S5012
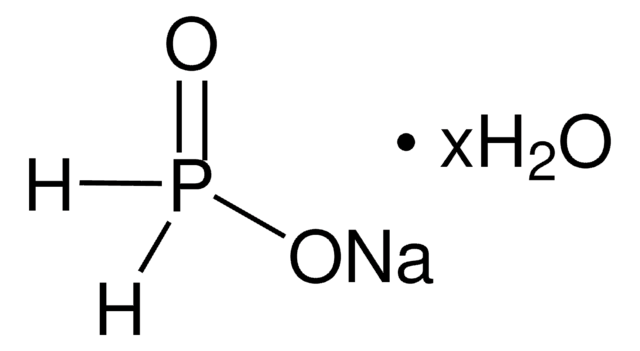
Sodium hypophosphite monohydrate
≥99%
Synonym(s):
Sodium hypophosphite 1-hydrate, Sodiumphosphinite
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NaH2PO2 · H2O
CAS Number:
Molecular Weight:
105.99
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder, crystals or chunks
solubility
water: 100 mg/mL, clear, colorless
SMILES string
O.[Na+].[O-][PH2]=O
InChI
1S/Na.H3O2P.H2O/c;1-3-2;/h;3H2,(H,1,2);1H2/q+1;;/p-1
InChI key
PLZNPHDJGFDNRM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Sodium hypophosphite monohydrate can be used as a:
- Reducing agent in the synthesis of nickel nanoparticles (NiNP) from nickel acetate tetrahydrate under microwave irradiation.
- Hydrogen donor in the enantioselective transfer hydrogenation of aliphatic and aromatic ketones to corresponding alcohols in presence of ruthenium catalyst.
- Catalyst in esterification of spent grain to improve heavy metal ions adsorption capacity using N,N-dimethylformamide (DMF) as a solvent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fast esterification of spent grain for enhanced heavy metal ions adsorption.
Li Q, et al.
Bioresource Technology, 101(10), 3796-3799 (2010)
Microwave assisted greener synthesis of nickel nanoparticles using sodium hypophosphite.
Eluri R and Paul B
Materials Letters, 76, 36-39 (2012)
Biphasic Glycerol/2-MeTHF, Ruthenium-Catalysed Enantioselective Transfer Hydrogenation of Ketones Using Sodium Hypophosphite as Hydrogen Donor.
Guyon C, et al.
European Journal of Organic Chemistry, 2013(24), 5439-5444 (2013)
Efficient large-scale radical deoxygenation in alcoholic solvents using sodium hypophosphite and a phase-transfer agent: application to erythromycins.
A E Graham et al.
The Journal of organic chemistry, 65(8), 2583-2585 (2000-05-02)
Radical reaction of sodium hypophosphite with terminal alkynes: synthesis of 1,1-bis-H-phosphinates.
Sonia Gouault-Bironneau et al.
Organic letters, 7(26), 5909-5912 (2005-12-16)
[reaction: see text] The room-temperature radical addition of sodium hypophosphite to terminal alkynes produces the previously unknown 1-alkyl-1,1-bis-H-phosphinates in moderate yield. The reaction is initiated by R3B and air and proceeds under mild conditions in an open container. The bissodium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service