P22389
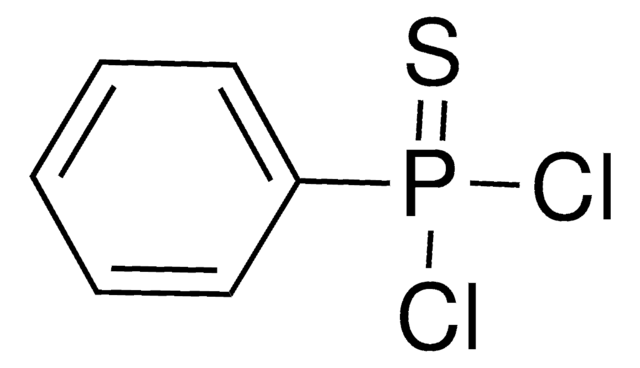
Phenyl dichlorophosphate
≥95%
Synonym(s):
Phenyl phosphodichloridate, Phenyl phosphorodichloridate, Phenyl phosphoryl dichloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5OP(O)Cl2
CAS Number:
Molecular Weight:
210.98
Beilstein:
511863
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
refractive index
n20/D 1.523 (lit.)
bp
241-243 °C (lit.)
density
1.412 g/mL at 25 °C (lit.)
SMILES string
ClP(Cl)(=O)Oc1ccccc1
InChI
1S/C6H5Cl2O2P/c7-11(8,9)10-6-4-2-1-3-5-6/h1-5H
InChI key
TXFOLHZMICYNRM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent for the preparation of phosphate diesters.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chun-Wei Kuo et al.
Molecules (Basel, Switzerland), 17(11), 13662-13672 (2012-11-22)
Upon treatment with phenyl dichlorophosphate (PhOP=OCl(2)) in acetonitrile at ambient temperature, a variety of ketoximes underwent a Beckmann rearrangement in an effective manner to afford the corresponding amides in moderate to high yields.
The Journal of Organic Chemistry, 53, 2077-2077 (1988)
Xiaoming Zhou et al.
Chemosphere, 235, 163-168 (2019-07-01)
Two novel phosphorus-containing copolyesters were synthesized by direct polycondensation reaction of phenyl dichlorophosphate, 1,4-succinic acid and 1,4-butanediol using stannous chloride (SnCl2) and 4-Methylbenzenesulfonic acid as catalyst, and its chemical structures were identified by 1H and 31P nuclear magnetic resonances (1H
Yvan Ecochard et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-15)
Epoxy materials have attracted attention for many applications that require fireproof performance; however, the utilization of hazardous reagents brings about potential damage to human health. Eugenol and cardanol are renewable, harmless resources (according to ECHA) that allow the achievement of
Dhruvkumar Soni et al.
Biomaterials, 222, 119441-119441 (2019-09-01)
While antiretroviral therapy (ART) has revolutionized treatment and prevention of human immunodeficiency virus type one (HIV-1) infection, regimen adherence, viral mutations, drug toxicities and access stigma and fatigue are treatment limitations. These have led to new opportunities for the development
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service