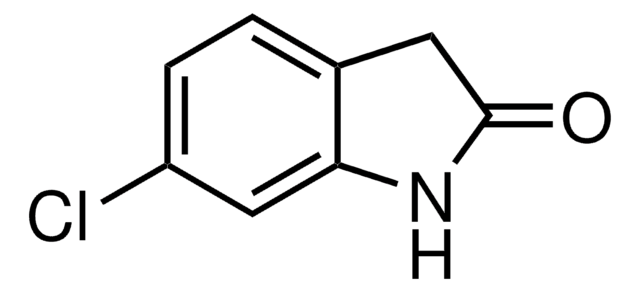
O9808
2-Oxindole
97%
Synonym(s):
2-Indolinone, Oxindole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H7NO
CAS Number:
Molecular Weight:
133.15
Beilstein:
114692
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
bp
227 °C/73 mmHg (lit.)
mp
123-128 °C (lit.)
SMILES string
O=C1Cc2ccccc2N1
InChI
1S/C8H7NO/c10-8-5-6-3-1-2-4-7(6)9-8/h1-4H,5H2,(H,9,10)
InChI key
JYGFTBXVXVMTGB-UHFFFAOYSA-N
Gene Information
human ... PGR(5241)
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Lanzi et al.
International journal of cancer, 85(3), 384-390 (2000-02-01)
ret-derived oncogenes are frequently and specifically expressed in thyroid tumors. In contrast to the ret receptor, ret oncoproteins are characterized by ligand-independent tyrosine-kinase activity and tyrosine phosphorylation. In this study, novel synthetic arylidene 2-indolinone compounds were evaluated as inhibitors of
Rahul R Khanwelkar et al.
Bioorganic & medicinal chemistry, 18(13), 4674-4686 (2010-06-24)
A series of new ureidoindolin-2-one derivatives were synthesized and evaluated as inhibitors of receptor tyrosine kinases. Investigation of structure-activity relationships at positions 5, 6, and 7 of the oxindole skeleton led to the identification of 6-ureido-substituted 3-pyrrolemethylidene-2-oxindole derivatives that potently
Yong-Jin Wua et al.
Current medicinal chemistry, 12(4), 453-460 (2005-02-22)
During the past five years, several members of the KCNQ potassium channel gene family have been identified with a high degree of CNS specificity. Within the KCNQ family, the combination of the KCNQ2/KCNQ3 proteins, and the KCNQ5/KCNQ3 arrangement has been
Morteza Bararjanian et al.
The Journal of organic chemistry, 75(9), 2806-2812 (2010-04-15)
An efficient palladium-catalyzed protocol for the synthesis of 3-arylidene-2-oxindoles has been developed. In this approach, a sequential one-pot six-component reaction via Ugi/Heck carbocyclization/Sonogashira/nucleophilic addition was used for the synthesis of the desired skeleton.
Fangrui Zhong et al.
Organic letters, 13(1), 82-85 (2010-12-09)
The first tertiary amine catalyzed enantioselective Morita-Baylis-Hillman (MBH) reaction of isatins with acrylates has been demonstrated, allowing asymmetric synthesis of biologically significant 3-substituted-3-hydroxy-2-oxindoles in good yields and with excellent enantioselectivities. The C6'-OH group of β-isocupreidine (β-ICD) is believed to facilitate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service