N32601
Norcamphor
98%
Synonym(s):
2-Norbornanone, Bicyclo[2.2.1]heptan-2-one
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H10O
CAS Number:
Molecular Weight:
110.15
Beilstein:
1209657
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
168-172 °C (lit.)
mp
93-96 °C (lit.)
SMILES string
O=C1C[C@@H]2CC[C@H]1C2
InChI
1S/C7H10O/c8-7-4-5-1-2-6(7)3-5/h5-6H,1-4H2/t5-,6+/m1/s1
InChI key
KPMKEVXVVHNIEY-RITPCOANSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J R Collins et al.
The Journal of biological chemistry, 263(7), 3164-3170 (1988-03-05)
The hydroxylations of d-camphor, norcamphor, pericyclocamphanone, and 5,5-difluorocamphor by cytochrome P-450cam have been examined using theoretical methods to identify and characterize properties which determine product specificity. Experimental results indicate that each molecule is hydroxylated with quite different regio-specificity when metabolized
J Contzen et al.
Biochemistry, 37(13), 4317-4324 (1998-04-29)
Step-scan time-resolved Fourier transform infrared spectroscopy with a time resolution of 5 micros was applied to the carbon monoxide complex of cytochrome P-450cam (CYP101) to study the bimolecular ligand-rebinding process after flash photolysis. Spectral changes in the CO ligand stretch
M B Bass et al.
Proteins, 13(1), 26-37 (1992-05-01)
While cytochrome P-450cam catalyzes the hydroxylation of camphor to 5-exo-hydroxycamphor with 100% stereospecificity, norcamphor is hydroxylated by this enzyme yielding 45% 5-exo-, 47% 6-exo-, and 8% 3-exo-hydroxynorcamphor (Atkins, W.M., Sligar, S.G., J. Am. Chem. Soc. 109:3754-3760, 1987). The present study
P J Loida et al.
The Journal of biological chemistry, 270(10), 5326-5330 (1995-03-10)
The stereoselectivity of cytochrome P450cam hydroxylation has been investigated with the enantiomerically pure substrate analog norcamphor. (1R)- and (1S)-norcamphor (> 92 enantiomeric excess) were characterized in the hydroxylation reaction with cytochrome P450cam with respect to the product profile, steady state
Marko D Mihovilovic et al.
ChemSusChem, 1(1-2), 143-148 (2008-07-09)
Recombinant Escherichia coli cells expressing monooxygenases of different bacterial origin were evaluated in microbial Baeyer-Villiger oxidations of racemic fused ketones. During the enzymatic oxidation process, both the "normal" lactone generated by migration of the more-substituted carbon atom and/or the "abnormal"
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service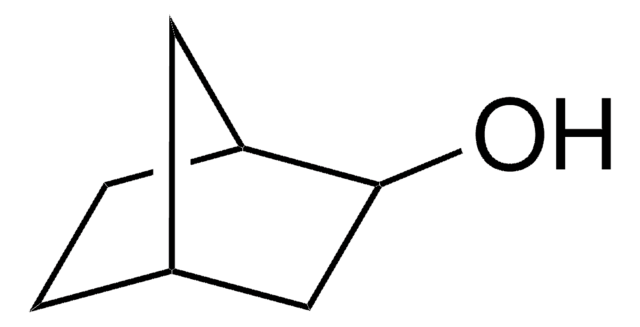





![Bicyclo[3.3.1]nonan-9-one ≥98%](/deepweb/assets/sigmaaldrich/product/structures/270/852/60661ded-13fb-4fc7-af36-a381880070a5/640/60661ded-13fb-4fc7-af36-a381880070a5.png)

![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)
