M57006
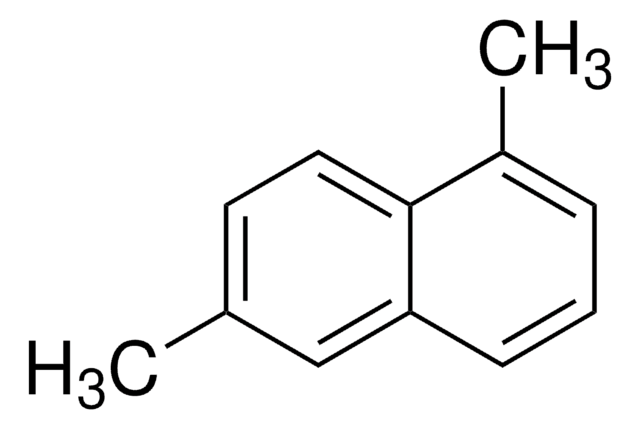
2-Methylnaphthalene (β)
95%
Synonym(s):
β-Methylnaphthalene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H7CH3
CAS Number:
Molecular Weight:
142.20
Beilstein:
906859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
241-242 °C (lit.)
mp
34-36 °C (lit.)
density
1 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc2ccccc2c1
InChI
1S/C11H10/c1-9-6-7-10-4-2-3-5-11(10)8-9/h2-8H,1H3
InChI key
QIMMUPPBPVKWKM-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544) , CYP2A6(1548)
mouse ... Cyp2a5(13087)
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Florin Musat et al.
Environmental microbiology, 11(1), 209-219 (2008-09-25)
The anaerobic biodegradation of naphthalene, an aromatic hydrocarbon in tar and petroleum, has been repeatedly observed in environments but scarcely in pure cultures. To further explore the relationships and physiology of anaerobic naphthalene-degrading microorganisms, sulfate-reducing bacteria (SRB) were enriched from
Kazutoshi Shindo et al.
Bioscience, biotechnology, and biochemistry, 75(3), 505-510 (2011-03-11)
We performed combinational bioconversion of substituted naphthalenes with PhnA1A2A3A4 (an aromatic dihydroxylating dioxygenase from marine bacterium Cycloclasticus sp. strain A5) and prenyltransferase NphB (geranyltransferase from Streptomyces sp. strain CL190) or SCO7190 (dimethylallyltransferase from Streptomyces coelicolor A3(2)) to produce prenyl naphthalen-ols.
Yu Wang et al.
Chemical communications (Cambridge, England), (8)(8), 1090-1091 (2005-02-19)
Using sodium deoxycholate as a protective medium, the selective recognition of Cu(II) at ng ml(-1) level is realized through dynamic phosphorescence quenching of 1-bromo-2-methylnaphthalene (BMN) without deoxygenation. The limit of detection is 4.32 ng ml(-1), and the relative standard deviation
Michael Safinowski et al.
Environmental microbiology, 8(2), 347-352 (2006-01-21)
The sulfate-reducing culture N47 can utilize naphthalene or 2-methylnaphthalene as the sole carbon source and electron donor. Here we show that the initial reaction in the naphthalene degradation pathway is a methylation to 2-methylnaphthalene which then undergoes the subsequent oxidation
Radosław Swiercz et al.
International journal of occupational medicine and environmental health, 23(4), 385-389 (2011-02-11)
The aim of the study was to evaluate the toxicokinetics of 2-methylnaphtalene (2-MN) during and after inhalation exposure. Male Wistar rats were exposed to 2-MN vapours at nominal concentrations of 200 or 400 mg/m3 in the dynamic inhalation chamber for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service