G10806
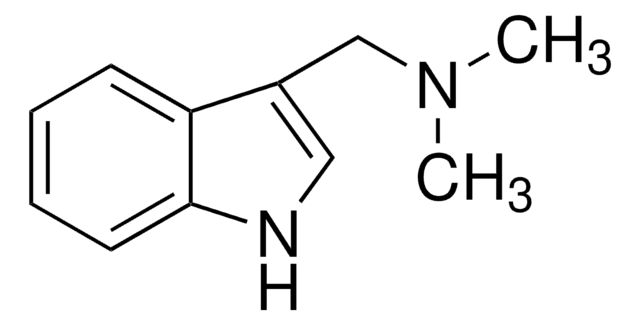
Gramine
97.5%
Synonym(s):
3-(Dimethylaminomethyl)indole, Donaxine, NSC 16892
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H14N2
CAS Number:
Molecular Weight:
174.24
Beilstein:
140521
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97.5%
mp
132-134 °C (lit.)
SMILES string
CN(C)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-13(2)8-9-7-12-11-6-4-3-5-10(9)11/h3-7,12H,8H2,1-2H3
InChI key
OCDGBSUVYYVKQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Dopamine D2 receptor antagonists
- Anti-malarial drugs
- 5-indolyl-Mannich bases
- Proliferation inhibitors
- Inhibitors of human mast cell chymase
- Preparation of DL-tryptophan
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- 3-vinylindoles
- Serotonin 5-HT6 receptor ligand templates
- Selective protein kinase c delta (PKCδ) down regulators
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
332.6 °F
Flash Point(C)
167 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Iwata et al.
European journal of pharmacology, 432(1), 63-70 (2001-12-06)
We examined the effects of 2,5,6-tribromo-1-methylgramine (TBG), isolated from bryozoan, and its derivative, 5,6-dibromo-1,2-dimethylgramine (DBG), on the contraction of rat aorta. TBG and DBG decreased the high-K(+)-induced increase in muscle contraction and cytosolic Ca(2+) level ([Ca(2+)](i)), respectively. The inhibitory effects
Brian Chauder et al.
Organic letters, 4(5), 815-817 (2002-03-01)
[reaction: see text] In the presence of NXS (X = Br, I, Cl), gramine derivatives 1, derived by combined directed ortho metalation (DoM)-cross-coupling sequences, rapidly undergo retro-Mannich fragmentation (2) to afford 3-halo indoles 3 in 37-88% yields. A conceptually new
E L Barker et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 19(12), 4705-4717 (1999-06-15)
Mutation of a conserved Asp (D98) in the rat serotonin (5HT) transporter (rSERT) to Glu (D98E) led to decreased 5HT transport capacity, diminished coupling to extracellular Na+ and Cl-, and a selective loss of antagonist potencies (cocaine, imipramine, and citalopram
N Nakahata et al.
European journal of pharmacology, 382(2), 129-132 (1999-10-21)
5,6-Dibromo-1,2-dimethylgramine evoked Ca(2+) release from skeletal muscle sarcoplasmic reticulum through ryanodine receptors in a concentration-dependent manner with an EC(50) of 22.2 microM. Since the EC(50) of caffeine was 0.885 mM, 5,6-dibromo-1,2-dimethylgramine was 40 times more sensitive than caffeine. Among 14
Robert M Williams et al.
Journal of the American Chemical Society, 125(40), 12172-12178 (2003-10-02)
The first total synthesis of paraherquamide A, a potent anthelmintic agent isolated from various Penicillium sp. with promising activity against drug-resistant intestinal parasites, is reported. Key steps in this asymmetric, stereocontrolled total synthesis include a new enantioselective synthesis of alpha-alkylated-beta-hydroxyproline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service