D116408
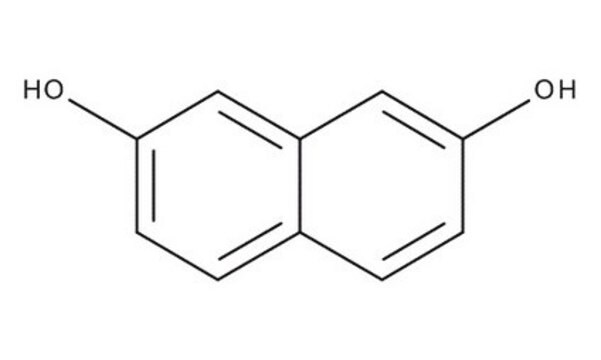
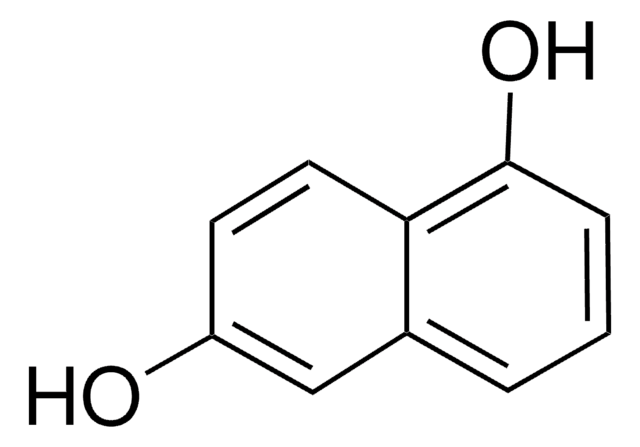
2,7-Dihydroxynaphthalene
97%
Synonym(s):
2,7-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
2042383
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
185-190 °C (lit.)
SMILES string
Oc1ccc2ccc(O)cc2c1
InChI
1S/C10H8O2/c11-9-3-1-7-2-4-10(12)6-8(7)5-9/h1-6,11-12H
InChI key
DFQICHCWIIJABH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,7-Dihydroxynaphthalene is a organic building block used to prepare sulfonic acids, divinylnaphthalenes, dyes, pigments, and fluorescent whiteners.
Application
Starting material for the synthesis of sulfonic acids and divinylnaphthalenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adriana Trapani et al.
Carbohydrate polymers, 207, 720-728 (2019-01-03)
The antibacterial activity of the S-unsubstituted- and S-benzyl-substituted-2-mercapto-benzothiazoles 1-4 has been evaluated after complexation with Methyl-β-Cyclodextrin (Me-β-CD) or incorporation in solid dispersions based on Pluronic® F-127 and compared with that of the pure compounds. This with the aim to gain
Raafat M Issa et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 62(4-5), 980-986 (2005-06-14)
The absorption spectra of mono- and bis-azo-derivatives obtained by coupling the diazonium salts of aromatic amines and 2,7-dihydroxynaphthalene have been studied in six organic solvents. The different absorption bands have been assigned and the effect of solvents on the charge
J. Chem. Soc. Perkin Trans. II, 721-721 (1993)
C R Bloom et al.
Biochemistry, 36(42), 12746-12758 (1997-10-23)
The binding of phenolic ligands to the insulin hexamer occurs as a cooperative allosteric process. Investigations of the allosteric mechanism from this laboratory resulted in the postulation of a model consisting of a three-state conformational equilibrium and the derivation of
Allergic contact dermatitis from 2,7-dihydroxynaphthalene in hair dye.
A Eskelinen et al.
Contact dermatitis, 36(6), 312-313 (1997-06-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service