A86406
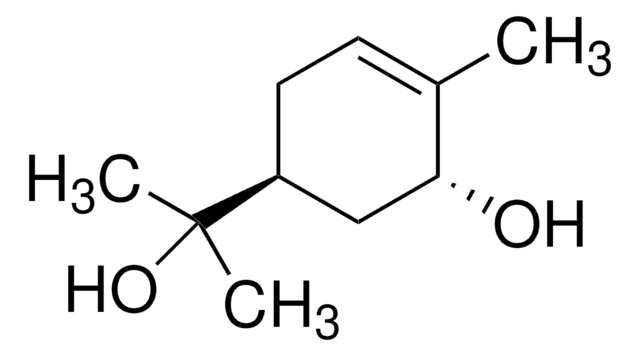
α-Angelica lactone
98%
Synonym(s):
alpha-Angelica lactone, 4-Hydroxy-3-pentenoic acid γ-lactone, 5-Methyl-2(3H)-furanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6O2
CAS Number:
Molecular Weight:
98.10
Beilstein:
108394
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.448 (lit.)
bp
55-56 °C/12 mmHg (lit.)
mp
13-17 °C (lit.)
density
1.092 g/mL at 25 °C (lit.)
SMILES string
CC1=CCC(=O)O1
InChI
1S/C5H6O2/c1-4-2-3-5(6)7-4/h2H,3H2,1H3
InChI key
QOTQFLOTGBBMEX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y M Ioannou et al.
Cancer research, 42(4), 1199-1204 (1982-04-01)
The effects of alpha-angelica lactone (alpha-AL), butylated hydroxyanisole (BHA), and beta-naphthoflavone (beta-NF) on the amount of benzo(alpha)pyrene (BP) metabolite:DNA adducts formed in the forestomach, lung, and liver of ICR/Ha mice were investigated 48 hr after p.o. administration of BP. BP
Lin Zhou et al.
Organic letters, 13(12), 3056-3059 (2011-05-21)
A direct highly diastereo- and enantioselective asymmetric vinylogous Mannich-type (AVM) reaction of aldimines with nonactivated natural α-angelica lactone has been successfully developed. It was demonstrated that the nonactivated natural α-angelica lactone is a useful vinylogous nucleophile to give the chiral
W A Nijhoff et al.
Carcinogenesis, 16(3), 607-612 (1995-03-01)
The naturally occurring anticarcinogens flavone and alpha-angelicalactone incorporated separately and simultaneously in the diet at 0.5, 0.1, 0.05 and 0.01% w/w, were studied with respect to their effects on oesophageal, gastric, intestinal, colonic and hepatic (i) glutathione S-transferase (GST) enzyme
Xin Huang et al.
Chemical communications (Cambridge, England), 48(18), 2439-2441 (2012-01-24)
The first organocatalytic asymmetric assembly of Morita-Baylis-Hillman carbonates of isatins and α-angelica lactone has been studied, affording multifunctional products containing two valuable pharmacophores and vicinal quaternary chiral centers in high stereoselectivity (up to 92% ee, dr >95:5).
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service