All Photos(4)
About This Item
Empirical Formula (Hill Notation):
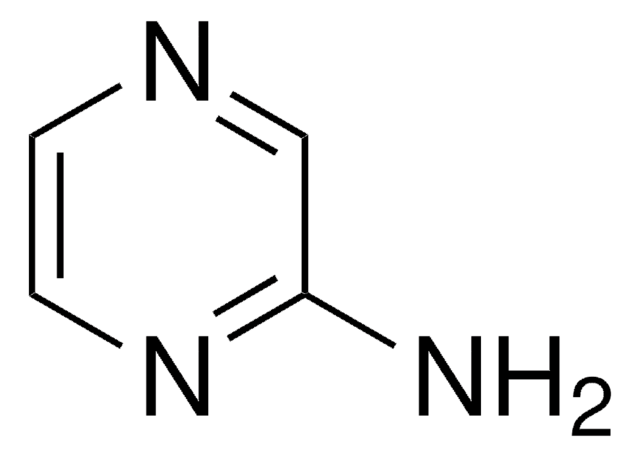
C5H6N2
CAS Number:
Molecular Weight:
94.11
Beilstein:
105692
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
flakes
bp
248 °C (lit.)
mp
60-63 °C (lit.)
SMILES string
Nc1cccnc1
InChI
1S/C5H6N2/c6-5-2-1-3-7-4-5/h1-4H,6H2
InChI key
CUYKNJBYIJFRCU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Elizabeta Sauer et al.
Antimicrobial agents and chemotherapy, 48(12), 4532-4541 (2004-11-25)
The utilization pathway for the uptake of NAD and nicotinamide riboside was previously characterized for Haemophilus influenzae. We now report on the cellular location, topology, and substrate specificity of PnuC. pnuC of H. influenzae is only distantly related to pnuC
Chao Fang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(4), 1588-1593 (2008-07-22)
Our algorithm [B. Tian, G. Wu, G. Liu, J. Chem. Phys. 87 (1987) 7300] is introduced to obtain the temporal bond polarizabilities of 2- and 3-aminopyridine from their Raman intensities, which supply fruitful electronic information of the nonresonant Raman excited
B Barna et al.
Acta biologica Hungarica, 50(1-3), 257-267 (1999-11-26)
The effect of GYKI 52466, a selective, non-competitive antagonist of the AMPA glutamate receptor subtype was investigated on the development, expression and propagation of 3-aminopyridine-induced cortical ictal activity, both in the primary and secondary focus. In one group of animals
Effects of chronic, intrauterine organic and inorganic mercury intoxication on the epileptogenicity of developing rat.
B Barna et al.
Central European journal of public health, 8 Suppl, 73-75 (2000-08-16)
Pierre Garcia et al.
Organic letters, 13(8), 2030-2033 (2011-03-19)
Bimolecular cobalt-catalyzed [2 + 2 + 2] cycloadditions between yne-ynamides and nitriles afford bicyclic 3- or 4-aminopyridines in up to 100% yield. The high regioselectivity observed depends on the substitution pattern at the starting ynamide. Aminopyridines bearing TMS and Ts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service