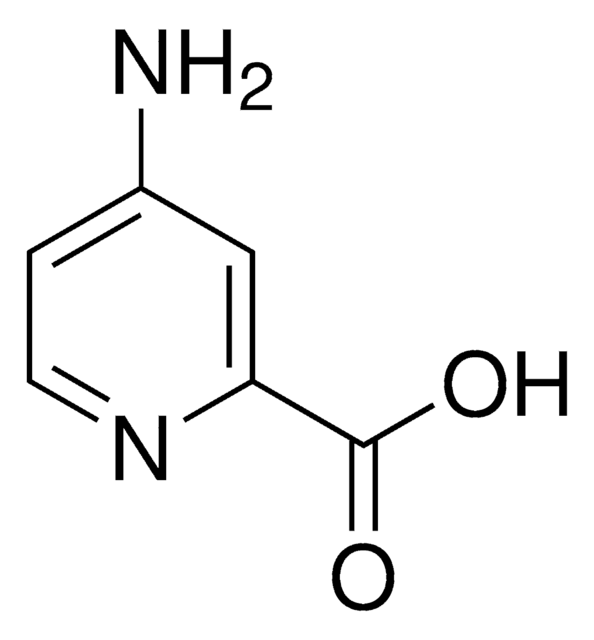
A68300
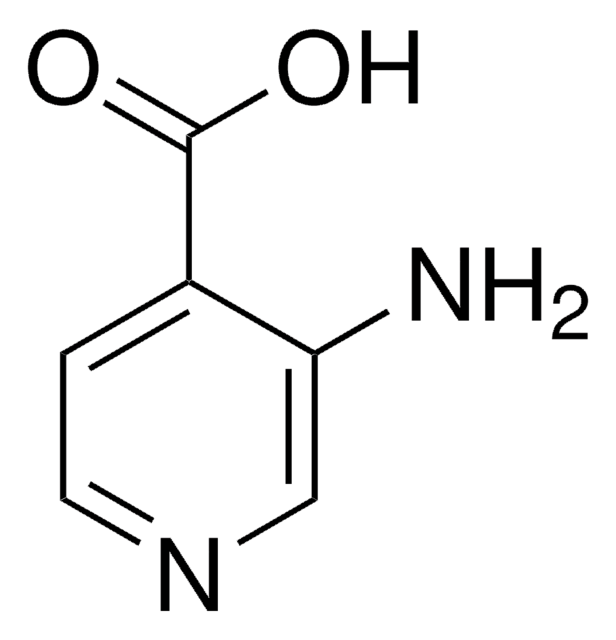
2-Aminopyridine-3-carboxylic acid
98%
Synonym(s):
2-Aminonicotinic acid, 2-Aminopyridine-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O2
CAS Number:
Molecular Weight:
138.12
Beilstein:
119031
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
295-297 °C (dec.) (lit.)
SMILES string
Nc1ncccc1C(O)=O
InChI
1S/C6H6N2O2/c7-5-4(6(9)10)2-1-3-8-5/h1-3H,(H2,7,8)(H,9,10)
InChI key
KPIVDNYJNOPGBE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Aminopyridine-3-carboxylic acid can be used as:
- A ligand to prepare copper(II)-organic coordination compounds.
- A reactant to prepare pyrido[2′,1′:2,3]imidazo[4,5-c]isoquinolines by reacting with trimethylsilyl cyanide and phthalaldehyde.
- A reactant to synthesize organo-soluble and thermally stable poly(thiourea-amide-imide) polymers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ronald Bartzatt
Drugs in R&D, 8(6), 363-372 (2007-10-30)
Nitrogen mustard (N-mustard) compounds are considered important anticancer drugs. Various transporting agents have been utilised to carry N-mustard groups including coumarins, amides, polyaromatic molecules and cycloalkyl structures. N-mustards act as bifunctional alkylating agents that induce cross-linking within DNA strands and
Synthesis, characterization, spectroscopic and electrochemical investigation of 2-aminopyridine-3-carboxylic acid copper (II) complexes with diimine
Srivastava AK, et al.
Chemical Data Collections, 24, 100272-100272 (2019)
Mehmet Karabacak et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 91, 83-96 (2012-03-01)
The experimental (UV-vis and FT-IR) and theoretical study of 2-aminonicotinic acid (C(6)H(6)N(2)O(2)) was presented in this work. The ultraviolet absorption spectrum of title molecule that dissolved in ethanol and water were examined in the range of 200-400 nm. The FT-IR
Synthesis of pyrido [2′, 1′: 2, 3] imidazo [4, 5-c] isoquinolines via a one-pot, three-component reaction
Maleki A and Rezayan AH
Tetrahedron Letters, 55(10), 1848-1850 (2014)
Novel thermally stable poly (thiourea-amide-imide) s bearing CS moieties and pyridine units in the backbone: Synthesis and properties
Kausar A, et al.
Polymer Degradation and Stability, 95(12), 2611-2618 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service