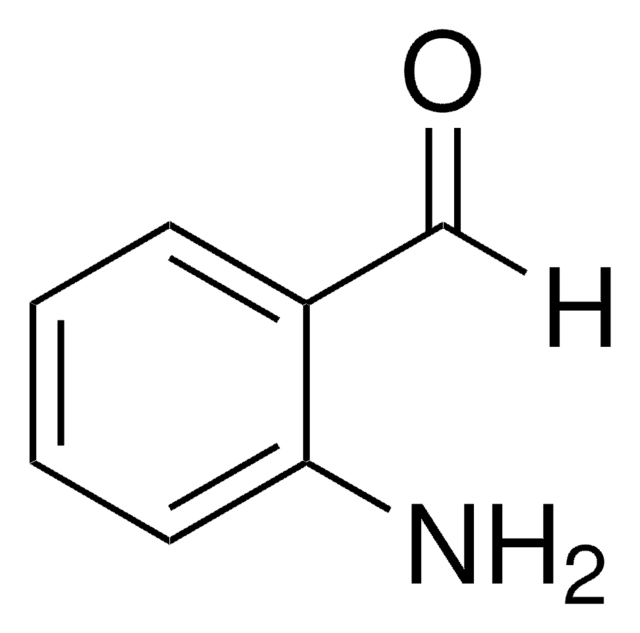
A38207
2-Aminoacetophenone hydrochloride
99%
Synonym(s):
ω-Aminoacetophenone hydrochloride, Phenacylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCH2NH2 · HCl
CAS Number:
Molecular Weight:
171.62
Beilstein:
3563173
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
194 °C (dec.) (lit.)
SMILES string
Cl.NCC(=O)c1ccccc1
InChI
1S/C8H9NO.ClH/c9-6-8(10)7-4-2-1-3-5-7;/h1-5H,6,9H2;1H
InChI key
CVXGFPPAIUELDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Anorexigenic activity of various derivatives of alpha-aminoacetophenone].
M S Sánchez et al.
Archivos de farmacologia y toxicologia, 5(3), 165-168 (1979-12-01)
M J Bossard et al.
The Journal of biological chemistry, 265(10), 5640-5647 (1990-04-05)
A mechanism for beta-chlorophenethylamine inhibition of dopamine beta-monooxygenase has been postulated in which bound alpha-aminoacetophenone is generated followed by an intramolecular redox reaction to yield a ketone-derived radical cation as the inhibitory species (Mangold, J.B., and Klinman, J.P. (1984) J.
Frank Sporkert et al.
Forensic science international, 133(1-2), 39-46 (2003-05-14)
A sensitive and reproducible method for the quantitative determination of cathinone (CTN), norpseudoephedrine (NPE, cathine) and norephedrine (NE) from hair was developed. The compounds were extracted for 4 hours with phosphate buffer pH 2.0, followed by a standard solid phase
J B Mangold et al.
The Journal of biological chemistry, 259(12), 7772-7779 (1984-06-25)
Functionalization of the beta-carbon of phenethylamines has been shown to produce a new class of substrate/inhibitor of dopamine beta-monooxygenase. Whereas both beta-hydroxy- and beta- chlorophenethylamine are converted to alpha-aminoacetophenone at comparable rates, only the latter conversion is accompanied by concomitant
Yasumasa Iwai et al.
Chemical & pharmaceutical bulletin, 50(3), 441-443 (2002-03-26)
The new coupling reaction of phenacylamines with silylstannane and lithium diisopropylamide (LDA) is reported. The treatment of a phenacylamine iodide 1 with (trimethylsilyl)tributylstannane (Me3SiSnBu3) and cesium fluoride (CsF) gave a dimerization product 2 having no iodine atom. Reaction of 1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![2-[(1R)-1-Aminoethyl]-phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/166/868/86b5a88f-7c6b-4634-aedc-c523a10e54aa/640/86b5a88f-7c6b-4634-aedc-c523a10e54aa.png)