91917
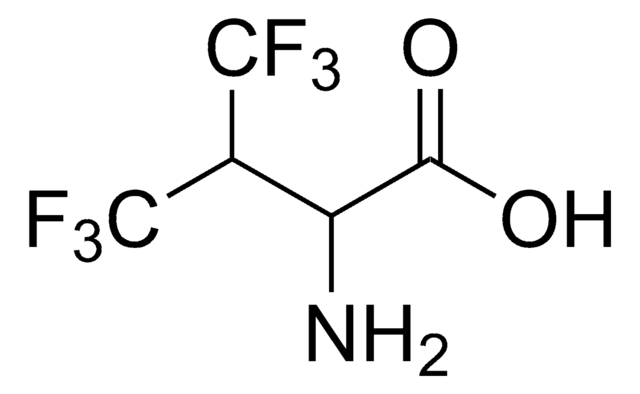
5,5,5-Trifluoro-DL-leucine
≥98.0% (sum of isomers, HPLC)
Synonym(s):
(±)-2-Amino-4-(trifluoromethyl)pentanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10F3NO2
CAS Number:
Molecular Weight:
185.14
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (sum of isomers, HPLC)
form
crystals
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
CC(CC(N)C(O)=O)C(F)(F)F
InChI
1S/C6H10F3NO2/c1-3(6(7,8)9)2-4(10)5(11)12/h3-4H,2,10H2,1H3,(H,11,12)
InChI key
XFGVJLGVINCWDP-UHFFFAOYSA-N
Related Categories
General description
5,5,5-Trifluoro-DL-leucine, also known as trifluoroleucine, is an analog of L-leucine amino acid, often used to synthesize highly fluorinated peptides.
Application
5,5,5-trifluoroleucine can be incorporated into the coiled-coil peptides to increase their thermal stability.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Takahiro Oba et al.
Bioscience, biotechnology, and biochemistry, 70(7), 1776-1779 (2006-07-25)
We characterized a trifluoroleucine-resistant mutant of Saccharomyces cerevisiae, TFL20, that has a mutation in the LEU4 gene. We monitored the concentration of extracellular i-AmOH and intracellular amino acids, and compared the ratios of gene expression in TFL20 with the wild-type
E Casalone et al.
Research in microbiology, 148(7), 613-623 (1998-10-10)
Eighteen mutants resistant to 5',5',5'-trifluoroleucine (TFL), a leucine analog, were isolated in Saccharomyces cerevisiae strains YNN281 and YNN282. The mutants were characterized genetically and clustered in two groups, one comprising all the dominant (TFL1) and the other one all the
Tatyana Panchenko et al.
Biotechnology and bioengineering, 94(5), 921-930 (2006-03-21)
Varied levels of fluorinated amino acid have been introduced biosynthetically to test the functional limits of global substitution on enzymatic activity and stability. Replacement of all the leucine (LEU) residues in the enzyme chloramphenicol acetyltransferase (CAT) with the analog, 5',5',5'-trifluoroleucine
C A Stella et al.
Molecular & general genetics : MGG, 262(2), 332-341 (1999-10-12)
Leucine uptake by Saccharomyces cerevisiae is mediated by three transport systems, the general amino acid transport system (GAP), encoded by GAP1, and two group-specific systems (S1 and S2), which also transport isoleucine and valine. A new mutant defective in both
A Niemz et al.
Journal of the American Chemical Society, 123(30), 7407-7413 (2001-07-27)
We have investigated the effect of trifluoroleucine substitution on the membrane-binding and tetramerization behavior of melittin. Analogues were synthesized in which Leu 9, Leu 13, and all four intrinsic leucine residues of melittin were replaced by 5,5,5-trifluoroleucine. Both the mono-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service