All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H12N2O5
CAS Number:
Molecular Weight:
252.22
UNSPSC Code:
12352208
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
206 °C
storage temp.
−20°C
SMILES string
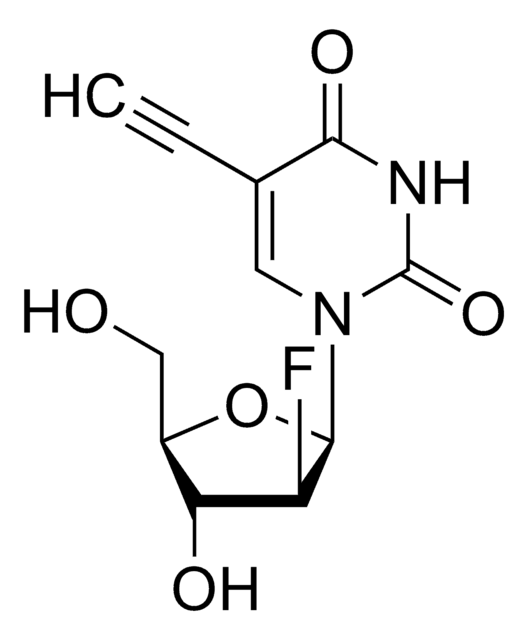
OC[C@@H]1[C@@H](O)C[C@H](N2C(NC(C(C#C)=C2)=O)=O)O1
InChI
1S/C11H12N2O5/c1-2-6-4-13(11(17)12-10(6)16)9-3-7(15)8(5-14)18-9/h1,4,7-9,14-15H,3,5H2,(H,12,16,17)/t7-,8+,9+/m0/s1
InChI key
CDEURGJCGCHYFH-DJLDLDEBSA-N
Related Categories
General description
5-Ethynyl-2′-deoxyuridine (EdU) is a thymidine analogue that can be incorporated into cellular DNA for cell proliferation studies. The incorporated nucleoside analogue can be detected by a copper-catalyzed click reaction with a fluorescent azide.
Application
5-Ethynyl-2′-deoxyuridine (EdU) is used for:
For a listing of the Baseclick kits see: Baseclick kits
- Labeling newly synthesized DNA during replication
- Cell proliferating assay studies
- Cell cycle analysis
For a listing of the Baseclick kits see: Baseclick kits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Evaluation of 5-ethynyl-2?-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system
C Zeng, et.al.
Brain Research, 1319, 21-32 (2010)
5-Ethynyl-2?-deoxycytidine as a new agent for DNA labeling: detection of proliferating cells
D Qu, et.al.
Analytical Biochemistry, 417, 112-121 (2011)
Synthesis, susceptibility to enzymatic phosphorylation, cytotoxicity and in vitro antiviral activity of lipophilic pyrimidine nucleoside/carborane conjugates.
Bialek-Pietras M, et al.
Journal of Organometallic Chemistry, 865, 166-172 (2018)
Cell type specific applicability of 5-ethynyl-2?-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry
S Diermeier
Cytometry. Part A : the Journal of the International Society For Analytical Cytology, 75, 535-546 (2009)
Site-directed spin-labeling of nucleic acids by click chemistry: detection of abasic sites in duplex DNA by EPR spectroscopy.
Jakobsen A, et al.
Journal of the American Chemical Society, 132(30), 10424-10428 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service