862126
3,4-Dehydro-DL-proline
98%
Synonym(s):
(±)-2,5-Dihydro-1H-pyrrole-2-carboxylic acid, (±)-3-Pyrroline-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
Beilstein:
471693
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
reaction suitability
reaction type: solution phase peptide synthesis
mp
245 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
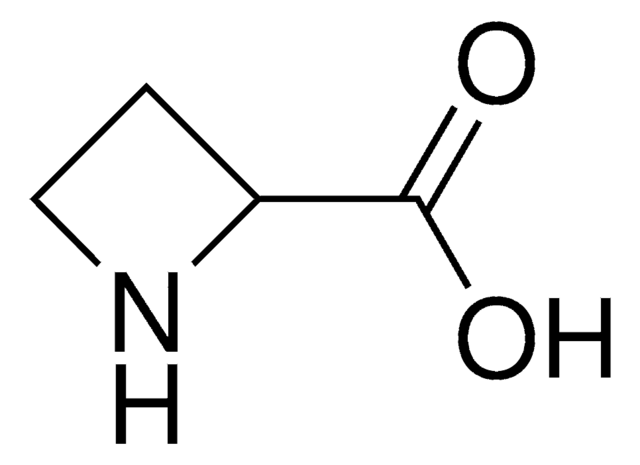
OC(=O)C1NCC=C1
InChI
1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h1-2,4,6H,3H2,(H,7,8)
InChI key
OMGHIGVFLOPEHJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Andrew G Mark et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(8), 1474-1480 (2011-04-28)
The expression of chirality at surfaces, arising from the adsorption of chiral molecules, is usually discussed in terms of the molecular handedness. However, the adsorption process often leads to a new manifestation of chirality in the form of the adsorption
M Bucher et al.
Plant molecular biology, 35(4), 497-508 (1998-02-12)
A differential screen of a tomato root hair cDNA library resulted in the cloning of two cDNAs, Dif10 and Dif54, whose corresponding genes are preferentially expressed in root hair cells as determined by analysis of mRNA levels in various tomato
H Jiang et al.
Scandinavian journal of immunology, 44(2), 101-107 (1996-08-01)
Complement subcomponent C1q has been recently implicated in the modulation of autocrine binding of TNF-alpha to murine macrophages for induction of nitric oxide synthase. In the present study, the putative role of C1q in increasing TNF-alpha binding to L929 cells
Zhaohui Meng et al.
Protein expression and purification, 49(1), 83-87 (2006-04-08)
Pyrroline-5-carboxylate reductase (P5CR) catalyzes the reduction of Delta1-pyrroline-5-carboxylate (P5C) to proline with concomitant oxidation of NAD(P)H to NAD(P)(+). The enzymatic cycle between P5C and proline is very important in many physiological and pathological processes. Human P5CR was over-expressed in Escherichia
Hyun-Ju Kim et al.
Journal of microbiological methods, 64(1), 17-26 (2005-06-02)
The gentamicin survival assay, a method routinely used to estimate bacterial infection of eukaryotic host cells, depends on the presumed limited penetration of gentamicin across the eukaryotic cell membrane. However, some studies have suggested that gentamicin may in fact enter
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service