76763
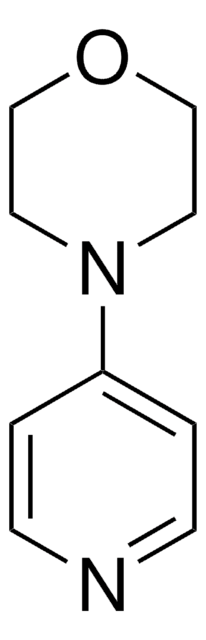
1-(4-Pyridyl)piperazine
≥97.0% (GC)
Synonym(s):
4-Piperazinopyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13N3
CAS Number:
Molecular Weight:
163.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
form
powder
impurities
≤1% water
mp
137-141 °C
SMILES string
C1CN(CCN1)c2ccncc2
InChI
1S/C9H13N3/c1-3-10-4-2-9(1)12-7-5-11-6-8-12/h1-4,11H,5-8H2
InChI key
OQZBAQXTXNIPRA-UHFFFAOYSA-N
Gene Information
rat ... Chrnb2(54239)
Related Categories
General description
1-(4-Pyridyl) piperazine (or 4-Piperazinopyridine) is an active structural component that is used as a building block to prepare various medicinally important active molecules.
Application
1-(4-Pyridyl) piperazine can be used as a building block for the synthesis of:
- Nocathiacin I analogs for antibacterial studies.
- 4-amino-pyridyl derivatives, benzimido isoquinoline based derivatives and tert-pentylphenoxyalkyl piperazine derivatives for various biological applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N ε-Acryloyllysine Piperazides as Irreversible Inhibitors of Transglutaminase 2: Synthesis, Structure?Activity Relationships, and Pharmacokinetic Profiling.
Wodtke R, et al.
Journal of Medicinal Chemistry, 61(10), 4528-4560 (2018)
Polymethacrylates containing a 4-amino-pyridyl derivative covalently attached as effective catalysts in acylation chemistry: self-activation by neighboring group effects
Mennenga T, et al.
Polymer International, 64(12), 1685-1689 (2015)
Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl) piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl) amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist.
Swanson D M, et al.
Journal of Medicinal Chemistry, 48(6), 1857-1872 (2005)
Synthesis and biological activity of novel tert-butyl and tert-pentylphenoxyalkyl piperazine derivatives as histamine H3R ligands.
Szczepanska K, et al.
European Journal of Medicinal Chemistry, 152, 223-234 (2018)
Selenium dioxide-mediated synthesis of ?-ketoamides from arylglyoxals and secondary amines
Shaw AY, et al.
Tetrahedron Letters, 53(32), 4151-4153 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service