73200
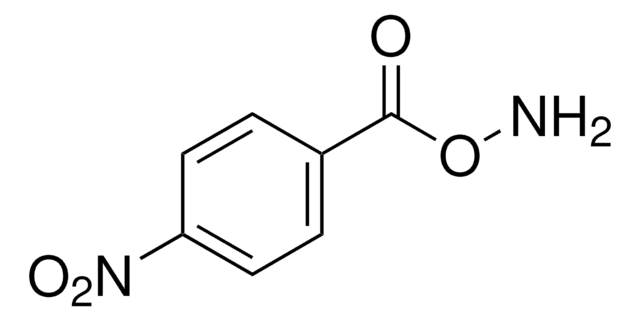
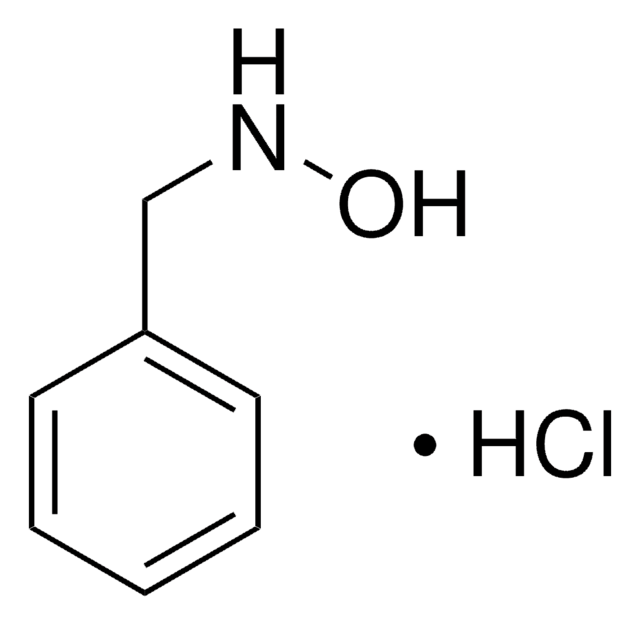
O-(4-Nitrobenzyl)hydroxylamine hydrochloride
≥98.5% (AT)
Synonym(s):
4-Nitrobenzyloxyamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4CH2ONH2 · HCl
CAS Number:
Molecular Weight:
204.61
Beilstein:
3709565
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.5% (AT)
form
crystals
mp
215 °C (dec.) (lit.)
functional group
nitro
SMILES string
Cl.NOCc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H8N2O3.ClH/c8-12-5-6-1-3-7(4-2-6)9(10)11;/h1-4H,5,8H2;1H
InChI key
LKCAFSOYOMFQSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Other Notes
Reagent for the preparation of N-(4-nitrobenzyloxy)-amino acids as substrates for an unambiguous N-hydroxypeptide synthesis
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Joo Hyun Lim et al.
Frontiers in microbiology, 9, 2333-2333 (2018-10-16)
2-Deoxy-scyllo-inosose (DOI) has been a valuable starting natural product for the manufacture of pharmaceuticals or chemical engineering resources such as pyranose catechol. DOI synthase, which uses glucose-6-phosphate (Glc6P) as a substrate for DOI biosynthesis, is indispensably involved in the initial
T D Traylor et al.
Journal of chromatography, 272(1), 9-20 (1983-01-14)
A new quantitative procedure for the high-performance liquid chromatographic (HPLC) resolution of human brain gangliosides employing reversed-phase chromatography is described. To provide a derivative which can be determined by UV absorption techniques, p-nitrobenzyloxyamine was coupled to the gangliosides. Derivatization involves
B X Chen et al.
Mutation research, 273(3), 253-261 (1992-05-01)
The abasic site is one of the most frequent changes occurring in DNA and has been shown to be lethal and mutagenic. An abasic site in DNA can be tagged by reaction with O-4-nitrobenzylhydroxylamine (NBHA), resulting in the formation of
M Pauly et al.
Carbohydrate research, 282(1), 1-12 (1996-02-28)
An improved procedure has been developed for the rapid derivatization of oligosaccharides with UV-detectable p-nitrobenzylhydroxylamine (PNB). The improved conditions used result in quantitative derivatization of neutral oligosaccharides. Sialylated oligosaccharides can also be quantitatively PNB-derivatized without detectable desialylation. Of the oligosaccharides
T. Kolasa et al.
Tetrahedron, 30, 3591-3591 (1974)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service