730300
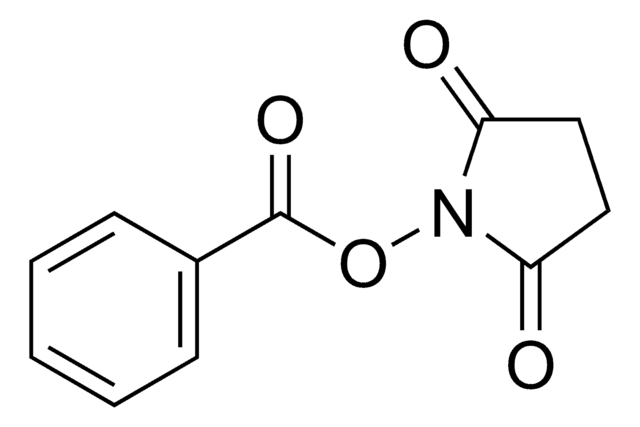
Methacrylic acid N-hydroxysuccinimide ester
98%
Synonym(s):
N-(Methacryloxy)succinimide, N-(Methacryloyloxy)succinimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H9NO4
CAS Number:
Molecular Weight:
183.16
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
mp
101-105 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)ON1C(=O)CCC1=O
InChI
1S/C8H9NO4/c1-5(2)8(12)13-9-6(10)3-4-7(9)11/h1,3-4H2,2H3
InChI key
ACGJEMXWUYWELU-UHFFFAOYSA-N
General description
Methacrylic acid N-hydroxysuccinimide ester(NHS-MA) belongs to the class of reactive monomers known as N-hydroxysuccinimide (NHS) esters. It is commonly used for functionalizing biomolecules through amine-reactive coupling reactions infields such as bioconjugation, protein labeling, and peptide synthesis. It also serves as a crosslinking agent or linker molecule for the conjugation of drugs or targeting ligands to carrier materials.
Application
Methacrylic acid N-hydroxysuccinimide ester can be used:
- As a monomer to prepare degradable amphiphilic diblock copolymer microparticles via RAFT polymerization, for low pH-triggered drug delivery. NHS-MA can shield the drug molecule from degradation, enhance its solubility, and improve its pharmacokinetic properties.
- For the surface functionalization of poly-ε-caprolactone (PCL) scaffolds used for tissue engineering. NHS groups are used to couple with chitosan of various molecular weights.
- To prepare biocompatible polymer hydrogel for enzymatic biofuel cells. The hydrogel can serve as an enzyme-immobilizing matrix for enzymatic bioelectrodes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrosprayed cysteine-functionalized degradable amphiphilic block copolymer microparticles for low pH-triggered drug delivery
Marie Finnegan, et al
Introduction to Polymer Chemistry, 10 (2019)
Postpolymerization modification of poly (pentafluorophenyl methacrylate): Synthesis of a diverse water-soluble polymer library
Gibson MI, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 47(17), 4332-4345 (2009)
A Modifiable, Spontaneously Formed Polymer Gel with Zwitterionic and N-Hydroxysuccinimide Moieties for an Enzymatic Biofuel Cell
Yixuan Huang, et al.
ACS applied polymer materials, 3, 631-639 (2021)
Theato, P.
Journal of Polymer Science Part A: Polymer Chemistry, 46, 6677-6677 (2008)
Fabrication of Unique Magnetic Bionanocomposite for Highly Efficient Removal of Hexavalent Chromium from Water
Zhong Y, et al.
Scientific reports, 6(17), 31090-31090 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service