678015
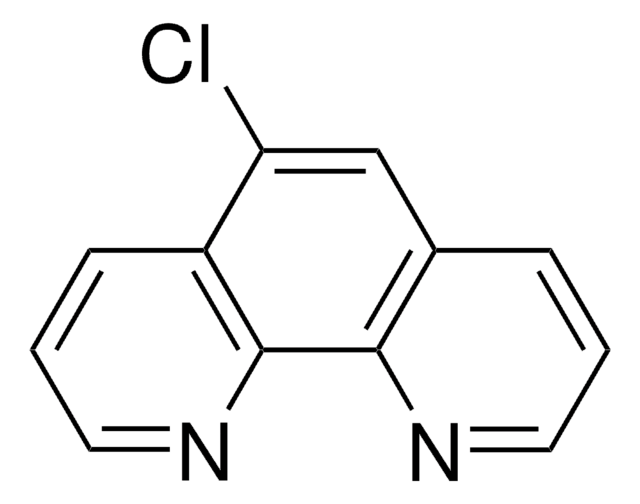
4,7-Dichloro-1,10-phenanthroline
97%
Synonym(s):
4,7-Dichloro-o-phenanthroline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H6Cl2N2
CAS Number:
Molecular Weight:
249.10
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
243-247 °C
SMILES string
Clc1ccnc2c1ccc3c(Cl)ccnc23
InChI
1S/C12H6Cl2N2/c13-9-3-5-15-11-7(9)1-2-8-10(14)4-6-16-12(8)11/h1-6H
InChI key
GIEQBYJCGYHHSU-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna M Maroń et al.
Dalton transactions (Cambridge, England : 2003), 49(14), 4441-4453 (2020-03-18)
Three Re(i) carbonyl complexes [ReCl(CO)3(Ln)] bearing 2,2'-bipyridine, 2,2':6',2''-terpyridine, and 1,10-phenanthroline functionalized with diphenylamine/or triphenylamine units (L1-L3) were synthesized to explore the impact of highly electron donating units appended to the imine ligand on the thermal and optoelectronic properties of Re(i)
Ryan A Altman et al.
Organic letters, 8(13), 2779-2782 (2006-06-16)
[reaction: see text] 4,7-Dimethoxy-1,10-phenanthroline (L) was found to be an efficient ligand for the copper-catalyzed N-arylation of imidazole with aryl iodides and bromides under mild conditions. A variety of hindered and functionalized imidazoles and aryl halides were transformed in good
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service