672068
Vinylphosphonic acid
≥90% (T)
Synonym(s):
Ethenephosphonic acid, Ethylenephosphonic acid, P-Ethenylphosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHP(O)(OH)2
CAS Number:
Molecular Weight:
108.03
Beilstein:
1741622
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥90% (T)
impurities
≤7.0% water
mp
36 °C (Lit. dry VPA) (lit.)
density
1.37 g/mL at 20 °C (lit.)
SMILES string
OP(O)(=O)C=C
InChI
1S/C2H5O3P/c1-2-6(3,4)5/h2H,1H2,(H2,3,4,5)
InChI key
ZTWTYVWXUKTLCP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Vinylphosphonic acid (VPA) can be used as a monomer unit for the synthesis of poly(vinylphosphonic acid) via free radical polymerization. It is also used to develop copolymers of VPA with acrylonitrile, N-isopropylacrylamide, styrene, vinylpyrrolidone, and acrylic and methacrylic acid. These copolymers find potential application in hydrogels, drug delivery, biomimetic mineralization, and polymer electrolyte membranes in fuel cells.
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
467.6 °F
Flash Point(C)
242 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Noh-Seok Kwak et al.
Journal of hazardous materials, 203-204, 213-220 (2012-01-03)
Poly(vinylphosphonic acid-co-methacrylic acid) microbeads were synthesized by suspension polymerization, and their indium adsorption properties were investigated. The obtained microbeads were characterized by Fourier transform infrared (FT-IR) spectroscopy and scanning electron microscopy (SEM). The microbeads were wrinkled spheres, irrespective of the
Synthesis, microstructure, and acidity of poly (vinylphosphonic acid)
Bingol B, et al.
Macromolecular Rapid Communications, 27(20), 1719-1724 (2006)
Richard D Bertram et al.
Biochemistry, 41(24), 7725-7731 (2002-06-12)
During the past 5 years a great deal of structural and biochemical information has given us a detailed insight into the molecular mechanism of action of the PcrA DNA helicase and challenged previous notions about the molecular mechanism of action
James D Kretlow et al.
Biomacromolecules, 11(3), 797-805 (2010-02-04)
Stimulus responsive materials hold great promise in biological applications as they can react to changes in physiological stimuli to produce a desired effect. Stimulus responsive macromers designed to respond to temperature changes at or around 37 degrees C and the
Single-step synthesis of styryl phosphonic acids via palladium-catalyzed Heck coupling of vinyl phosphonic acid with aryl halides
McNichols BW, et al.
Chemical Communications (Cambridge, England), 53(92), 12454-12456 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service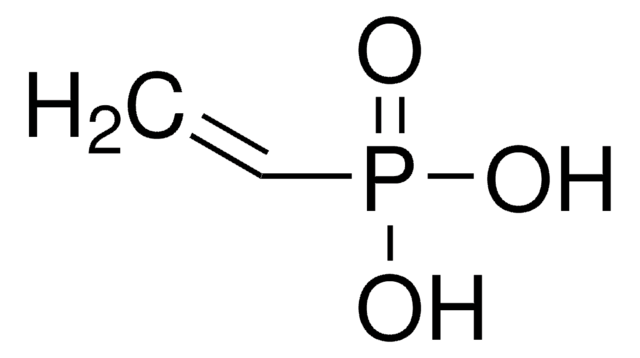



![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)