570362
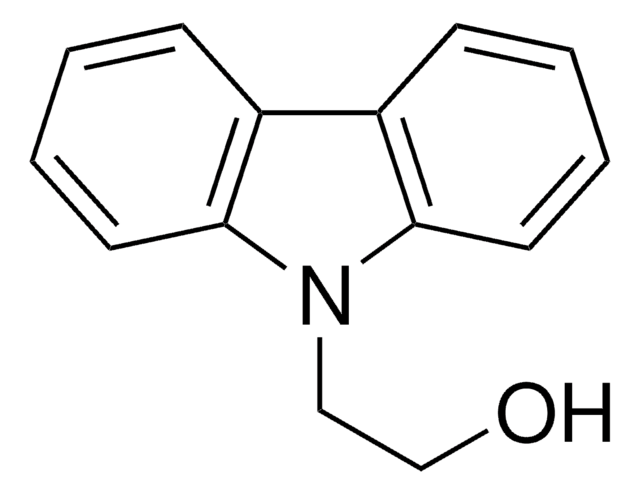
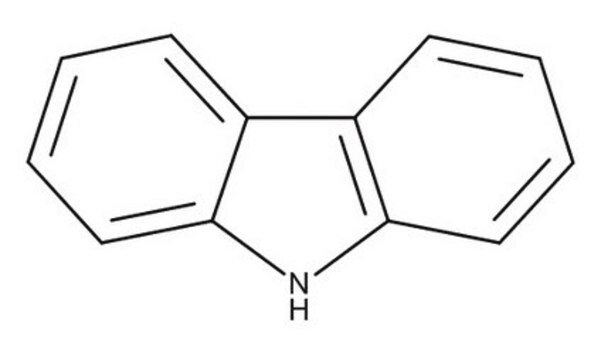
2-(9H-Carbazol-9-yl)ethyl acrylate
97%
Synonym(s):
2-(9H-Carbazol-9-yl)ethyl methacrylate, 9H-Carbazole-9-ethylacrylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H15NO2
CAS Number:
Molecular Weight:
265.31
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
mp
77-81 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC(=O)OCCn1c2ccccc2c3ccccc13
InChI
1S/C17H15NO2/c1-2-17(19)20-12-11-18-15-9-5-3-7-13(15)14-8-4-6-10-16(14)18/h2-10H,1,11-12H2
InChI key
KOHMQTTZAWPDNE-UHFFFAOYSA-N
Related Categories
General description
2-(9H-Carbazol-9-yl)ethyl acrylate (PCz) is an acrylate based conducting polymer with carbazole as electron donating pendant group. It can be used as a charge transporting material as it exhibits high charge carrier mobility and photochemical stability. It also has high thermal and electroluminescent properties that make it useful in organic electronics based application.
Application
PCz can be used as a conducting polymer in the fabrication of a variety of devices which include organic light emitting diodes (OLEDs), photovoltaic cells and memory based devices.
Features and Benefits
NLO chromophore
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Non-volatile WORM memory device based on an acrylate polymer with electron donating carbazole pendant groups
Teo EYH, et al.
Organic Electronics, 7(3), 173-180 (2006)
Reversible addition-fragmentation chain transfer polymerization of methacrylates containing hole-or electron-transporting groups
Zhao P, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 45(2), 242-252 (2007)
Synthesis and switching properties of photochromic carbazole-spironaphthoxazine copolymer
Wang S, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 192(1), 17-22 (2007)
Poly(methyl methacrylate)copolymers containing pendant carbazole and oxadiazole moieties for applications in single-layer organic light emitting devices.
Evanoff DD, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 46(23), 7882-7897 (2008)
Single component transparent free-standing films based on polyhedral octasilicate-core dendrimers bearing carbazole terminal groups and their emission properties
Irie Y and Naka K
Journal of Polymer Science Part A: Polymer Chemistry, 54(5), 628-633 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service