569313
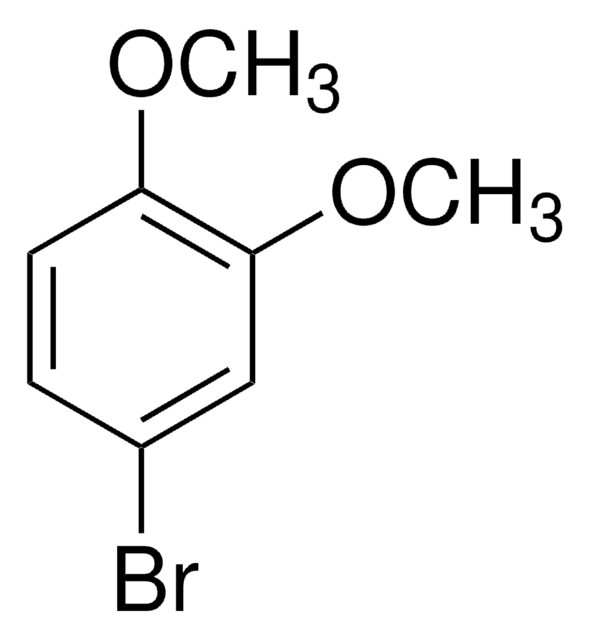
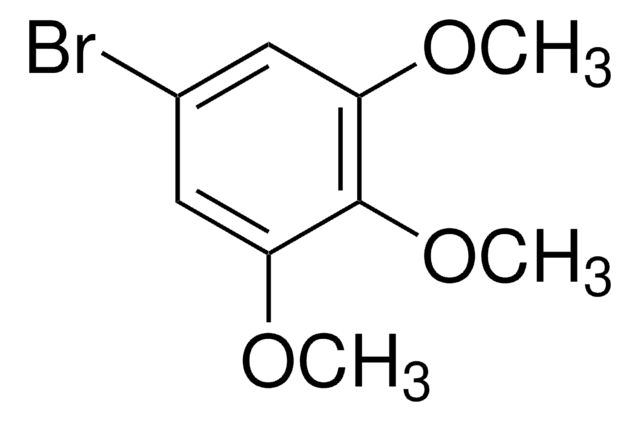
1-Bromo-3,5-dimethoxybenzene
97%
Synonym(s):
3,5-Dimethoxy-1-bromobenzene, 3,5-Dimethoxybromobenzene, 3,5-Dimethoxyphenyl bromide, 5-Bromo-1,3-dimethoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H3(OCH3)2
CAS Number:
Molecular Weight:
217.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
62-66 °C (lit.)
functional group
bromo
SMILES string
COc1cc(Br)cc(OC)c1
InChI
1S/C8H9BrO2/c1-10-7-3-6(9)4-8(5-7)11-2/h3-5H,1-2H3
InChI key
KRWRFIMBWRVMKE-UHFFFAOYSA-N
Related Categories
General description
1-Bromo-3,5-dimethoxybenzene can be synthesized by using 1,3-dimethoxybenzene via iridium-catalyzed arene borylation.
Application
1-Bromo-3,5-dimethoxybenzene may be used to synthesize the following:
- bis-[di-(3,5-dimethoxyphenyl)methylcyclopentadienyl]titanium(IV)dichloride,{η5 - C5H4-CH-[C6H4-(OCH3)2]2)2TiCl2 which can be used as an anti-cancer drug and is obtained via a multistep reaction process
- 5-bromo-benzene-1,3-diol via demethylation with boron tribromide(BBr3)
- 1-bromo-3,5-dimethoxy-2-nitrobenzene via nitration reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Diarylmethyl substituted titanocenes: promising anti-cancer drugs"
Pampillon C, et al.
Polyhedron, 25(10), 2101-2108 (2006)
"Novel CYP17 inhibitors: Synthesis, biological evaluation, structure?activity relationships and modelling of methoxy-and hydroxy-substituted methyleneimidazolyl biphenyls"
Hille.U, et al.
European Journal of Medicinal Chemistry, 44(7), 2765-2775 (2009)
"Photoswitching azo compounds in vivo with red light"
Samanta S, et al.
Journal of the American Chemical Society, 135(26), 9777- 9784 (2013)
Takashi Iijima et al.
Bioscience, biotechnology, and biochemistry, 73(11), 2547-2548 (2009-11-10)
An efficient synthesis of tri-O-methylated resveratrol is presented using an advanced Heck reaction promoted by Pd(dba)(2) in the presence of P(t-Bu)(3).
Ryan T Pekarek et al.
Journal of the American Chemical Society, 140(41), 13223-13232 (2018-10-04)
The design and fabrication of stable and efficient photoelectrochemical devices requires the use of multifunctional structures with complex heterojunctions composed of semiconducting, protecting, and catalytic layers. Understanding charge transport across such devices is challenging due to the interplay of bulk
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service