558028
(S)-(+)-4-Penten-2-ol
95%
Synonym(s):
(2S)-4-Penten-2-ol, (2S)-Pent-4-en-2-ol, (S)-(+)-Pent-4-en-2-ol, (S)-(-)-Pent-4-en-2-ol, (S)-4-Penten-2-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
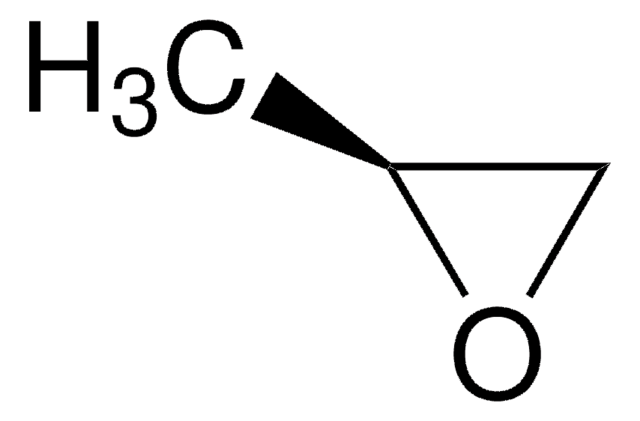
H2C=CHCH2CH(OH)CH3
CAS Number:
Molecular Weight:
86.13
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
optical activity
[α]20/D +5.0°, c = 1% in chloroform
refractive index
n20/D 1.4240 (lit.)
bp
115-116 °C (lit.)
density
0.837 g/mL at 25 °C (lit.)
functional group
allyl
hydroxyl
SMILES string
C[C@H](O)CC=C
InChI
1S/C5H10O/c1-3-4-5(2)6/h3,5-6H,1,4H2,2H3/t5-/m0/s1
InChI key
ZHZCYWWNFQUZOR-YFKPBYRVSA-N
Application
(S)-(+)-4-Penten-2-ol can be used:
- As an intermediate in one of the key synthetic steps for the preparation of undecenolide (−)-cladospolide C.
- To prepare a carboxamide derivative as a key intermediate for the preparation of (−)-iso-cladospolide B1.
- To prepare S-enantiomers of hydroxyeicosa tetraenoic acid named 19-HETE.
- To prepare an acryloyl ester derivative by reacting with acryloyl chloride, which is a key intermediate for the synthesis of parasorbic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
78.0 °F - closed cup
Flash Point(C)
25.56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantioselective total synthesis of iso-cladospolide B, cladospolide C and cladospolide B from tartaric acid
Prasad KR and Gandi VR
Tetrahedron Asymmetry, 22(5), 499-505 (2011)
A practical, stereospecific route to 18-, 19-, and 20-hydroxyeicosa-5 (Z), 8 (Z), 11 (Z), 14 (Z)-tetraenoic acids (18-, 19-, and 20-HETEs)
Gopal VR, et al.
Tetrahedron Letters, 45(12), 2563-2565 (2004)
A Chemoenzymatic Total Synthesis of the Undecenolide (−)-Cladospolide C
Banwell MG, et al.
Australian Journal of Chemistry, 58(7), 511-516 (2005)
Asymmetric synthesis of goniothalamin, hexadecanolide, massoia lactone, and parasorbic acid via sequential allylboration-esterification ring-closing metathesis reactions
Ramachandran PV, et al.
Tetrahedron Letters, 41(5), 583-586 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service