530336
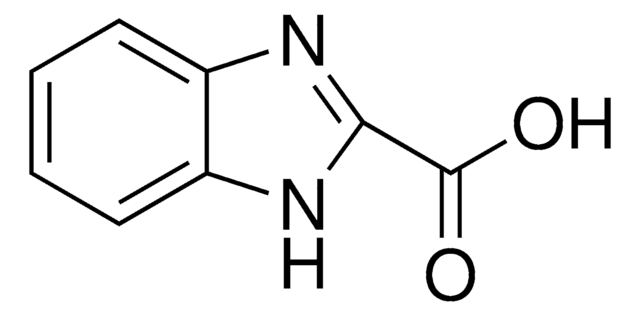
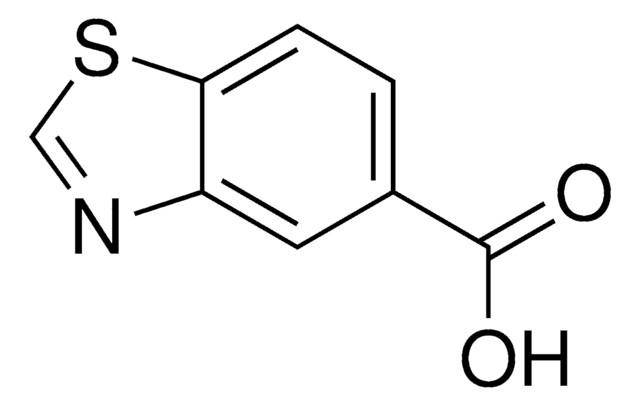
Benzothiazole-6-carboxylic acid
96%
Synonym(s):
1,3-Benzothiazole-6-carboxylic acid, Benzo[d]thiazole-6-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H5NO2S
CAS Number:
Molecular Weight:
179.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
245-251 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc2ncsc2c1
InChI
1S/C8H5NO2S/c10-8(11)5-1-2-6-7(3-5)12-4-9-6/h1-4H,(H,10,11)
InChI key
DMPZJACLHDWUFS-UHFFFAOYSA-N
General description
Benzothiazole-6-carboxylic acid (BTCA) is a benzothiazole derivative.
Application
Benzothiazole-6-carboxylic acid may be used in the synthesis of N-(pyridin-4-yl)benzo[d]thiazole-6-carboxamide, which shows the potential to inhibit K1 capsule formation in uropathogenic Escherichia coli. It may also be used as an internal standard during the quantification of benzothiazoles by liquid chromatography-electrospray ionization-tandem mass spectrometry.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
James W. Noah et al.
Probe Reports from the NIH Molecular Libraries Program, 2012 Dec 17 (Updated 2013 Apr 5) (2013-07-09)
Uropathogenic
Yoshihiro Sawada et al.
Pest management science, 59(1), 25-35 (2003-02-01)
The N'-benzoyl group of N-tert-butyl-N'-benzoyl-3,5-dimethylbenzohydrazide (1) was converted to a series of benzoheterocyclecarbonyl groups in order to investigate the potential usefulness of superimposing a hydrazine insecticide on 20-hydroxyecdysone. A series of analogues with benzodioxole, benzodioxane, benzodioxapine, indole, benzoxazole, benzoxazine or
Stefan Weiss et al.
Analytical chemistry, 77(22), 7415-7420 (2005-11-16)
The first method for the determination of commonly used corrosion inhibitors in environmental water samples by liquid chromatography-electrospray ionization-tandem mass spectrometry is presented. Benzotriazole (BTri) and the two isomers of tolyltriazole (5- and 4-TTri) are separated in an isocratic run.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![5-Bromo-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/408/367/d023f137-f9d5-4e3a-bc3a-89b109181588/640/d023f137-f9d5-4e3a-bc3a-89b109181588.png)