All Photos(1)
About This Item
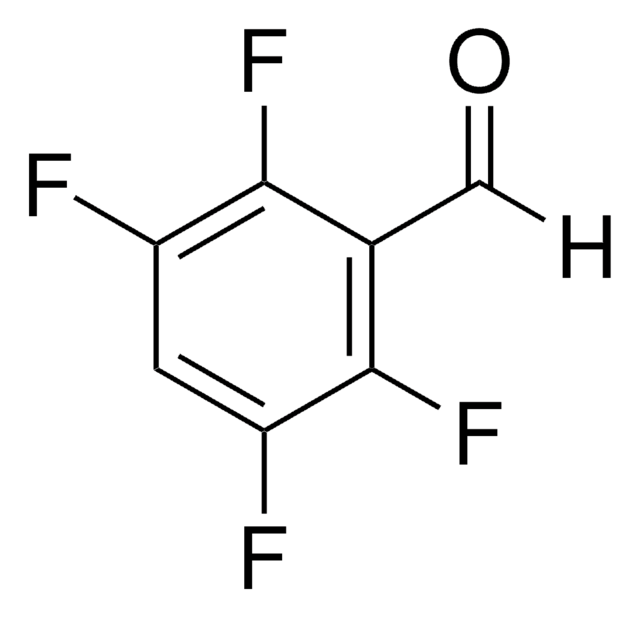
Linear Formula:
F3C6H2CHO
CAS Number:
Molecular Weight:
160.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.482 (lit.)
bp
168 °C (lit.)
density
1.408 g/mL at 25 °C (lit.)
SMILES string
Fc1cc(F)c(C=O)cc1F
InChI
1S/C7H3F3O/c8-5-2-7(10)6(9)1-4(5)3-11/h1-3H
InChI key
CYIFJRXFYSUBFW-UHFFFAOYSA-N
Application
2,4,5-Trifluorobenzaldehyde may be used in the synthesis of:
- bis(2,4,5-trifluorophenyl)methanone
- 8-phenyl-7-tosyl-1-(2,4,5-trifluorophenyl)octahydro-1H-pyrano[3,4-c] pyridine
- (E)-4-methoxy-N′-(2,4,5-trifluorobenzylidene)-benzohydrazide monohydrate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aminol initiated Prins cyclization for the synthesis of octahydro-1H-pyrano [3, 4-c] pyridine and hexahydro-1H-furo [3, 4-c] pyrrole derivatives.
Tetrahedron Letters, 55(34), 4817-4821 (2014)
(E)-4-Methoxy-N'-(2, 4, 5-trifluorobenzylidene) benzohydrazide monohydrate.
Maheswari R, et al.
IUCrJ, 1(5), x160846-x160846 (2016)
Zachary R Woydziak et al.
Synthesis, 46(2), 158-164 (2014-06-11)
Fluorinated fluorophores are valuable tools for studies of biological systems. However, amine-reactive single-isomer derivatives of these compounds are often very expensive. To provide an inexpensive alternative, we report a practical synthesis of 4-carboxy-Pennsylvania Green methyl ester. Derivatives of this hydrophobic
Zachary R Woydziak et al.
The Journal of organic chemistry, 77(1), 473-481 (2011-11-25)
Fluorination of fluorophores can substantially enhance their photostability and improve spectroscopic properties. To facilitate access to fluorinated fluorophores, bis(2,4,5-trifluorophenyl)methanone was synthesized by treatment of 2,4,5-trifluorobenzaldehyde with a Grignard reagent derived from 1-bromo-2,4,5-trifluorobenzene, followed by oxidation of the resulting benzyl alcohol.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service