All Photos(2)
About This Item
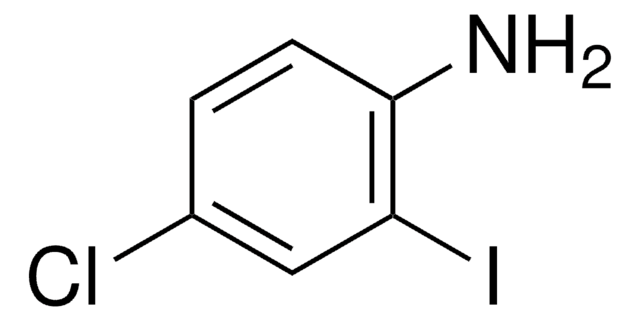
Linear Formula:
BrC6H3(I)NH2
CAS Number:
Molecular Weight:
297.92
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
69-72 °C (lit.)
functional group
bromo
iodo
SMILES string
Nc1ccc(Br)cc1I
InChI
1S/C6H5BrIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
InChI key
HHTYEQWCHQEJNV-UHFFFAOYSA-N
General description
4-Bromo-2-iodoaniline is a 2-iodoaniline derivative. It can be prepared by reacting 4-bromoaniline with iodine.
Application
4-Bromo-2-iodoaniline may be used in the following:
- Preparation of quinolone derivatives.
- Synthesis of a resin-bound sulfonamide, which was used as a starting material for the preparation of 2,3,5-trisubstituted indoles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shaei Huang et al.
Bioorganic & medicinal chemistry letters, 16(22), 5907-5912 (2006-09-23)
Through a comparison of X-ray co-crystallographic data for 1 and 2 in the Chek1 active site, it was hypothesized that the affinity of the indolylquinolinone series (2) for Chek1 kinase would be improved via C6 substitution into the hydrophobic region
Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines.
Cortese NA, et al.
The Journal of Organic Chemistry, 43(15), 2952-2958 (1978)
ON SOME HALOGEN DERIVATIVES OF AROMATIC AMINES AND THEIR ANALYSIS. I. 1.
Dains FB, et al.
Journal of the American Chemical Society, 40(6), 930-936 (1918)
T Y Wu et al.
Organic letters, 3(24), 3827-3830 (2001-11-27)
2,3,5-Trisubstituted indoles are synthesized in three steps starting from resin-bound aniline 2. R1 is introduced by a palladium-mediated coupling of the aryl iodide with terminal alkynes followed by intramolecular cyclization to form the indole core. Acylation at C-3 with an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service