All Photos(1)
About This Item
Empirical Formula (Hill Notation):
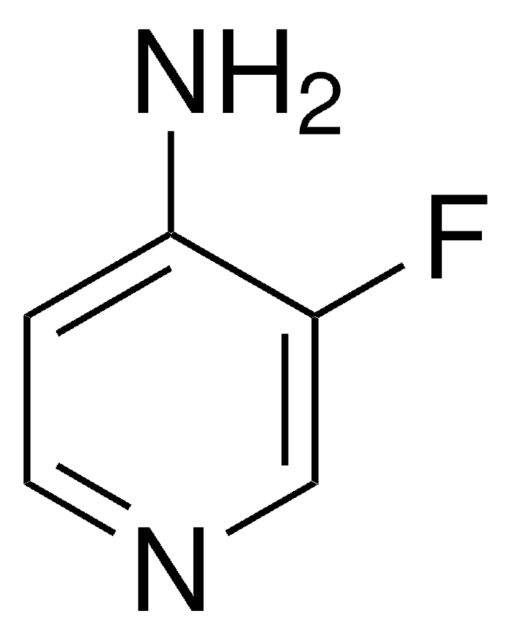
C5H5ClN2
CAS Number:
Molecular Weight:
128.56
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
90-94 °C (lit.)
functional group
chloro
SMILES string
Nc1ccnc(Cl)c1
InChI
1S/C5H5ClN2/c6-5-3-4(7)1-2-8-5/h1-3H,(H2,7,8)
InChI key
BLBDTBCGPHPIJK-UHFFFAOYSA-N
General description
4-Amino-2-chloropyridine is a chloroaminoheterocyclic compound. It readily undergoes Suzuki-Miyaura coupling with phenylboronic acid.
Application
4-Amino-2-chloropyridine may be used in the preparation of:
- 3-deazacytosine
- 7-substituted 1-alky-1,4-dihydro-4-oxo-1,6-naphthyridine-3-carboxylic acids
- 1,6-naphthyridines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pyridone-carboxylic Acids as Antibacterial Agents. I. Synthesis and Antibacterial Activity of 1-Alkyl-1, 4-dihydro-4-oxo-1, 8-and 1, 6-naphthyridine-3-carboxylic Acids.
Hirose T, et al.
Chemical & Pharmaceutical Bulletin, 30(7), 2399-2409 (1982)
Preparation and biological activity of various 3-deazapyrimidines and related nucleosides.
A Bloch et al.
Journal of medicinal chemistry, 16(3), 294-297 (1973-03-01)
A highly active catalyst for Suzuki-Miyaura cross-coupling reactions of heteroaryl compounds.
Kelvin L Billingsley et al.
Angewandte Chemie (International ed. in English), 45(21), 3484-3488 (2006-04-28)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service