All Photos(1)
About This Item
Linear Formula:
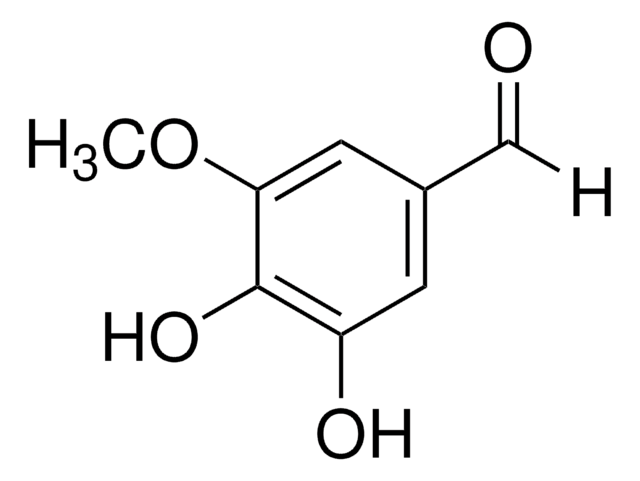
(HO)3C6H2CHO
CAS Number:
Molecular Weight:
154.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
223-225 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1cc(O)c(O)cc1O
InChI
1S/C7H6O4/c8-3-4-1-6(10)7(11)2-5(4)9/h1-3,9-11H
InChI key
WNCNWLVQSHZVKV-UHFFFAOYSA-N
General description
2,4,5-Trihydroxybenzaldehyde (2,4,5-THBA) is a tri-substituted benzaldehyde that can be prepared from sesamol. Its free radical-quenching ability, antioxidant bioactivity and cytotoxicity have been assessed. 2,4,5-THBA has been identified as one of the components in the ethyl acetate extract of Beta vulgaris var. cicla seeds. The freezing point, boiling point, density and refractive index of 2,4,5-THBA are 499.65K, 510.61K, 1.3725g/cm3 and 1.6400, respectively.
Application
2,4,5-Trihydroxybenzaldehyde may be used in the synthesis of:
- 2,4,5-tribenzyloxybenzaldehyde
- 2,4,5-trihydroxybenzaldehyde N-(diphenylmethylene)hydrazine
- 2-hydroxy-4,5-dimethoxybenzaldehyde
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephen J Mills et al.
Chembiochem : a European journal of chemical biology, 9(11), 1757-1766 (2008-06-25)
Novel benzene polyphosphates were synthesised as inositol polyphosphate mimics and evaluated against type-I inositol 1,4,5-trisphosphate 5-phosphatase, which only binds soluble inositol polyphosphates, and against the PH domain of protein kinase Balpha (PKBalpha), which can bind both soluble inositol polyphosphates and
Lorenzo Gennari et al.
Phytochemical analysis : PCA, 22(3), 272-279 (2011-02-22)
Beta vulgaris var. cicla (BV) leaves contain chemopreventive compounds that have been investigated for new drug discovery. These compounds belong to the family of the apigenin-glycosides. Since the leaves are seasonal products containing high percentages of water, they are easily
Ahluwalia VK.
Intermediates for Organic Synthesis, 51-51 (2010)
T H Tseng et al.
Toxicology, 161(3), 179-187 (2001-04-12)
As part of our earlier search for new compounds with improved biological activities including antioxidant, anti-inflammatory, and tumor growth inhibition activities, we synthesized 2,4,5-trihydroxybenzaldehyde (2,4,5-THBA) from commercially available Sesamol. First we examined the free radical-quenching capacity of 2,4,5-THBA, 3,4-dihydroxybenzaldehyde (3,4-DHBA)
Khalid Mohammed Khan et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 9(4), 588-595 (2012-11-16)
Benzophenonehydrazone Schiff bases 1-25 were synthesized and their in vitro antiglycation potential has been studied. Out of twenty-five compounds, thirteen showed varying degrees of antiglycation activity with IC50 values ranging between 25.7 - 305 μM, if compared with the standard
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service