All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.518 (lit.)
bp
65 °C/15 mmHg (lit.)
density
1.083 g/mL at 25 °C (lit.)
SMILES string
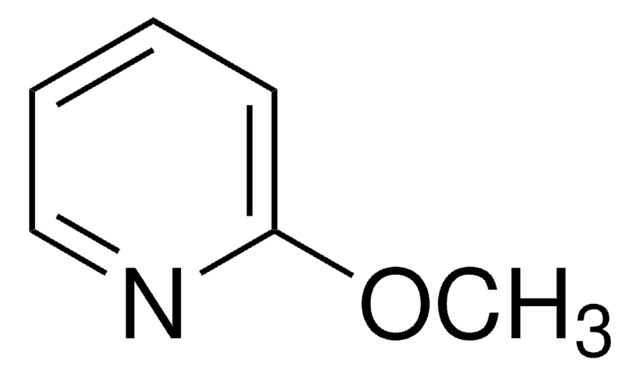
COc1cccnc1
InChI
1S/C6H7NO/c1-8-6-3-2-4-7-5-6/h2-5H,1H3
InChI key
UMJSCPRVCHMLSP-UHFFFAOYSA-N
Related Categories
General description
3-Methoxypyridine is a substituted pyridine. Kinetics of the oxidation of 3-methoxypyridine mediated by sulphate radicals has been investigated by flash photolysis of peroxodisulphate, S2O82-. Ortho lithiation of 3-methoxypyridine has been studied using mesityllithium as the metalating base.
Application
3-Methoxypyridine may be employed as a catalyst for the addition reaction of various 1,2-acyclic diones to activated acetylenic esters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
María L Dell'arciprete et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 8(17), 2498-2505 (2007-10-25)
The kinetics of the oxidation of pyridine, 3-chloropyridine, 3-cyanopyridine, 3-methoxypyridine and 3-methylpyridine mediated by SO4 (<M->) radicals are studied by flash photolysis of peroxodisulphate, S2O8(2-), at pH 2.5 and 9. The absolute rate constants for the reactions of both, the
Abhilash N Pillai et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(19), 5851-5860 (2008-05-10)
A systematic study of the addition of various 1,2-acyclic diones to activated acetylenic esters catalyzed by pyridine under mild conditions is described. This reaction provides a new protocol for the stereoselective synthesis of 1,2-diaroyl maleates. The exclusive formation of the
Ortho lithiation of 2-, 3-, and 4-methoxypyridines.
Comins DL and LaMunyon DH.
Tetrahedron Letters, 29(7), 773-776 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service