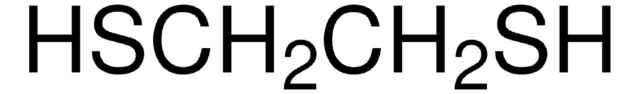467979
1,3-Dithiolane
97%
Synonym(s):
1,3-Dithiacyclopentane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6S2
CAS Number:
Molecular Weight:
106.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.599 (lit.)
bp
183 °C (lit.)
density
1.235 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
C1CSCS1
InChI
1S/C3H6S2/c1-2-5-3-4-1/h1-3H2
InChI key
IMLSAISZLJGWPP-UHFFFAOYSA-N
General description
1,3-Dithiolane ,a sulfur containing heterocyclic building block, is a cyclic thioether. Fragmentation modes of 1,3-oxathiolane under electron-impact have been investigated.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Differentiation between carbonyls and acetals in 1, 3-dithiane and 1, 3-dithiolane synthesis catalyzed by organotin triflates.
Sato T, et al.
The Journal of Organic Chemistry, 58(18), 4971-4978 (1993)
Ionization and dissociation of cyclic ethers and thioethers by electron-impact. A comparison between 1, 3-dioxolane, 1, 3-dithiolane and 1, 3-oxathiolane.
Conde-Caprace G and Collin JE.
Org. Mass Spectrom., 6(4), 415-423 (1972)
Elemental fluorine. Part 5. Reactions of 1, 3-dithiolanes and thioglycosides with fluorine-iodine mixtures.
Chambers RD, et al.
Journal of the Chemical Society. Perkin Transactions 1, 16, 1941-1944 (1996)
I M Hussaini et al.
Acta pharmacologica Sinica, 21(10), 897-904 (2001-08-15)
To investigate the effect of a group of novel synthetic dithiolane analogs of lignans and a well characterized platelet-activating factor (PAF) receptor antagonist, L659,989 on PAF-receptor binding, IFN-gamma- and lipopolysaccharide (LPS)-induced NO production, and steady-state inducible nitric-oxide synthase (iNOS) mRNA
Sylvie Goncalves et al.
Chemical communications (Cambridge, England), 46(31), 5778-5780 (2010-07-06)
A concise and diastereoselective formal total synthesis of triptolide, a natural product with a wide range of biological properties, is described. The key reaction is an unprecedented 6-endo-Trig cationic cyclization of a 2-alkenyl-1,3-dithiolane precursor induced by TMSOTf as Lewis acid.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service