464481
2-Octyl-1-dodecanol
97%
Synonym(s):
Octyldodecanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
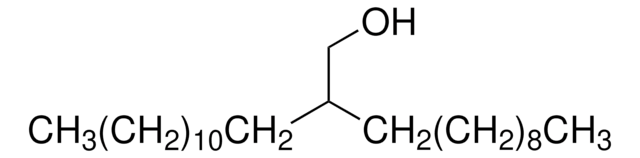
CH3(CH2)9CH[(CH2)7CH3]CH2OH
CAS Number:
Molecular Weight:
298.55
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.453 (lit.)
bp
234-238 °C/33 mmHg (lit.)
mp
−1-1 °C (lit.)
density
0.838 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCC(CO)CCCCCCCC
InChI
1S/C20H42O/c1-3-5-7-9-11-12-14-16-18-20(19-21)17-15-13-10-8-6-4-2/h20-21H,3-19H2,1-2H3
InChI key
LEACJMVNYZDSKR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Octyl-1-dodecanol is a branched alcohol.
Application
2-Octyl-1-dodecanol may be used to investigate its interaction with the hexameric capsules of resorcin[4]arene. It has been used as diluent in a extractant screening study for the recovery of putrescine (butylene-1,4-diamine, BDA) and cadaverine (pentylene-1,5-diamine, PDA) from aqueous solutions (fermentation broths) by liquid-liquid extraction.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
370.4 °F - open cup
Flash Point(C)
188 °C - open cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nancy Sharma et al.
Artificial cells, nanomedicine, and biotechnology, 45(3), 409-413 (2016-03-26)
The objective of present work was to enquire the potential use of embelin-loaded nanolipid carriers for brain targeting. The average particle size and polydispersity index (PDI) of optimized formulation (F19) were found to be 152 ± 19.7 nm and 0.143 ± 0.023, respectively. Nanolipid carrier
Extractive recovery of aqueous diamines for bio-based plastics production.
Krzyzaniak A, et al.
Journal of Chemical Technology and Biotechnology, 88(10), 1937-1945 (2013)
R S Lanigan
International journal of toxicology, 20 Suppl 3, 51-59 (2002-01-05)
Octyldodecyl Stearoyl Stearate functions as an occlusive skin-conditioning agent and as a nonaqueous viscosity-increasing agent in many cosmetic formulations. Current concentrations of use are between 0.7% and 23%, although historically higher concentrations were used. The chemical is formed by a
Contact sensitivity to octyldodecanol and trometamol in an anti-itch cream.
M Singh et al.
Contact dermatitis, 56(5), 289-290 (2007-04-20)
Strontium removal by new alkyl phenylphosphonic acids in supported liquid membranes with strip dispersion.
Ho WSW and Wang W.
Industrial & Engineering Chemistry Research, 41(3), 381-388 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service