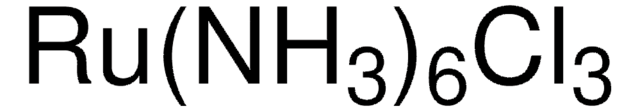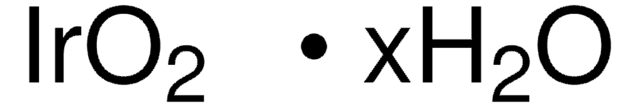450162
Potassium hexachloroiridate(IV)
99.99% trace metals basis
Synonym(s):
Iridium potassium chloride
About This Item
Recommended Products
Quality Level
Assay
99.99% trace metals basis
form
powder
impurities
≤150.0 ppm Trace Metal Analysis
mp
>385 °C (lit.)
SMILES string
[K+].[K+].Cl[Ir--](Cl)(Cl)(Cl)(Cl)Cl
InChI
1S/6ClH.Ir.2K/h6*1H;;;/q;;;;;;+4;2*+1/p-6
InChI key
WEKZJZDULNNSCD-UHFFFAOYSA-H
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Voltammetry at HMT-PMBI-coated glassy carbon electrodes: This study explores the electrochemical properties of potassium hexachloroiridate(IV) and its potential for sensitive detection, which could be valuable in analytical chemistry applications (M Rees et al., 2020).
- Antioxidant Total Capacity Assay: Introduces a novel assaying method for antioxidant capacity using potassium hexachloroiridate(IV), which could have implications in pharmacology and drug discovery (Y Liu et al., 2024).
- Mass Transfer in 3D-printed Electrolyzers: Examines the role of potassium hexachloroiridate(IV) in enhancing mass transfer in electrolytic systems, which is pivotal for chemical engineering and energy applications (SJC Weusten et al., 2021).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service