All Photos(1)
About This Item
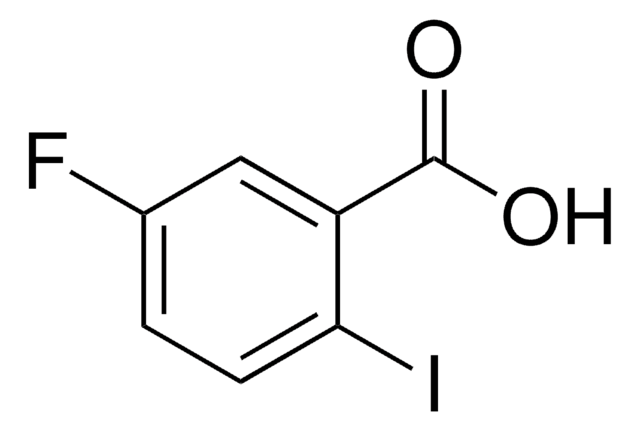
Linear Formula:
FC6H3(I)CO2H
CAS Number:
Molecular Weight:
266.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
123-126 °C (lit.)
functional group
carboxylic acid
fluoro
iodo
SMILES string
OC(=O)c1c(F)cccc1I
InChI
1S/C7H4FIO2/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3H,(H,10,11)
InChI key
CYCXAPWOBWWNRK-UHFFFAOYSA-N
General description
2-Fluoro-6-iodobenzoic acid is loose white crystal. It can be synthesized by using 2-amino-6-fluorobenzoic acid as the starting material.
Application
2-Fluoro-6-iodobenzoic acid may be used for the synthesis of fluoro-substituted benzoyl chlorides. It may be used for the one-pot regioselective synthesis of isocoumarins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 2-fluoro-6-iodobenzoic acid.
Zhao H-Y, et al.
Chemical Reagents, 11, 028-028 (2010)
Peri fluoro steric effects: syntheses and comparative acid-catalyzed isomerization of the 8-, 9-, and 11-fluoro-1, 2, 3, 4-tetrahydro-7, 12-dimethylbenz [a] anthracenes to exo methylene tautomers.
Witiak DT, et al.
The Journal of Organic Chemistry, 53(2), 345-352 (1988)
Regioselective One-Pot Synthesis of Isocoumarins and Phthalides from 2-Iodobenzoic Acids and Alkynes by Temperature Control.
Kumar MR, et al.
Advanced Synthesis & Catalysis, 355(16), 3221-3230 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service