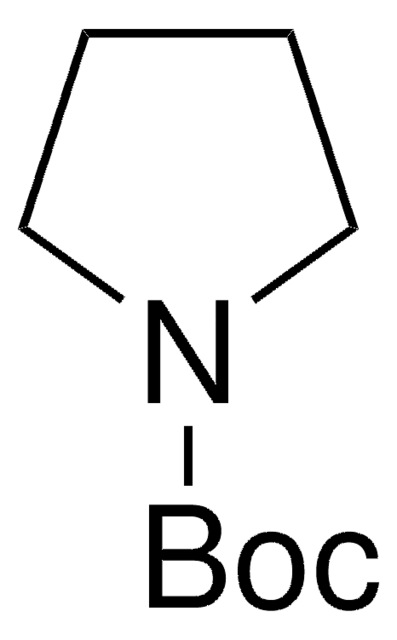
425834
N-Boc-pyrrole
98%
Synonym(s):
tert-Butyl 1-pyrrolecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13NO2
CAS Number:
Molecular Weight:
167.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.4685 (lit.)
bp
91-92 °C/20 mmHg (lit.)
density
1 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)OC(=O)n1cccc1
InChI
1S/C9H13NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H,1-3H3
InChI key
IZPYBIJFRFWRPR-UHFFFAOYSA-N
General description
N-Boc-pyrrole is an N-protected pyrrole. It undergoes Diels–Alder reaction with enantiomerically pure allene-1,3-dicarboxylates to form endo-adducts with retention in configurations at two newly generated stereogenic centers. It also undergoes cyclopropanation with methyl phenyldiazoacetate to form both monocyclopropane and dicyclopropane. Its Ir-catalyzed C-H borylation followed by cross coupling with 3-chlorothiophene to form biheterocycle has been reported.
Application
N-Boc-pyrrole was used in the synthesis of 1-(tert-butoxycarbonyl)-1H-pyrrol-2-ylboronic acid by treating with n-BuLi and subsequent reaction with trimethyl borate.
It may be used as starting material in the synthesis of the following:
It may be used as starting material in the synthesis of the following:
- tropane drivatives
- N-boc-2-(4-methoxyphenyl)pyrrole
- N-boc-pyrrol-2-ylboronic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
167.0 °F - closed cup
Flash Point(C)
75 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Recent progress in the synthesis of five-membered heterocycle boronic acids and esters.
Primas N, et al.
Tetrahedron, 66(41), 8121-8136 (2010)
Nikola Basarić et al.
Organic & biomolecular chemistry, 3(15), 2755-2761 (2005-07-21)
Two fluorescent off-on Ca2+ indicators based on APTRA (o-aminophenol-N,N,O-triacetic acid) as low-affinity ligand for Ca2+ and BODIPY(4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a fluorophore were synthesized. The new BODIPY-APTRA compounds absorb in the visible spectrum, with absorption maxima from 505 nm to 570 nm
Huw M L Davies et al.
Chemical Society reviews, 38(11), 3061-3071 (2009-10-23)
The metal catalyzed reactions of diazo compounds have been broadly used in organic synthesis. The resulting metal-carbenoid intermediates are capable of undergoing a range of unconventional reactions, and due to their high energy, they are ideal for initiating cascade sequences
Synthetic approaches to enantiomerically pure 8-azabicyclo [3.2. 1] octane derivatives.
Pollini GP, et al.
Chemical Reviews, 106(6), 2434-2454 (2006)
Venkata A Kallepalli et al.
The Journal of organic chemistry, 74(23), 9199-9201 (2009-11-10)
Ir-catalyzed C-H borylation is found to be compatible with Boc protecting groups. Thus, pyrroles, indoles, and azaindoles can be selectively functionalized at C-H positions beta to N. The Boc group can be removed on thermolysis or left intact during subsequent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service