412287
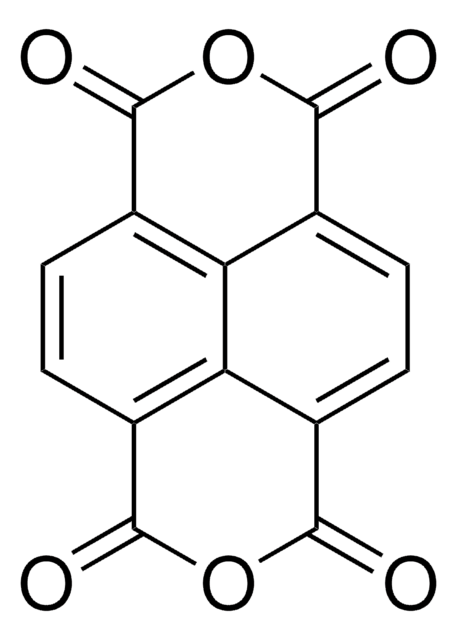
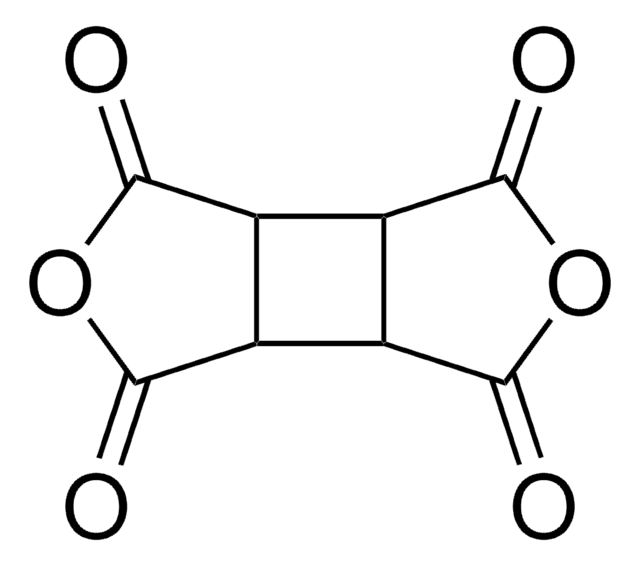
Pyromellitic dianhydride
97%
Synonym(s):
Benzene-1,2,4,5-tetracarboxylic dianhydride, PMDA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H2O6
CAS Number:
Molecular Weight:
218.12
Beilstein:
213583
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
powder
bp
397-400 °C (lit.)
mp
283-286 °C (lit.)
SMILES string
O=C1OC(=O)c2cc3C(=O)OC(=O)c3cc12
InChI
1S/C10H2O6/c11-7-3-1-4-6(10(14)16-8(4)12)2-5(3)9(13)15-7/h1-2H
InChI key
ANSXAPJVJOKRDJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyromellitic dianhydride is a dianhydride class of monomers commonly used in the preparation of polyimides polymers which are used in aerospace, electronics, and automotive industries because of their excellent thermal stability, high mechanical strength, and inherent flame resistance. It is also used in the synthesis of thermoplastic polymers such as polyesters and polyethers, as well as plasticizers and epoxy resins. Additionally, PMDA serves as a curing agent for epoxy resins. Epoxy resins are widely used in adhesives, coatings, composites, and electrical insulation materials.
Pyromellitic dianhydride (PMDA) is an acidic anhydride that can be used as a repair agent and as a chain extender in the formation of polyethylene terephthalate (PET) based chain extensions. It is mainly used in the production of thermoplastics and other coating applications.
Pyromellitic dianhydride (PMDA) is an acidic anhydride that can be used as a repair agent and as a chain extender in the formation of polyethylene terephthalate (PET) based chain extensions. It is mainly used in the production of thermoplastics and other coating applications.
Application
PMDA can be used as:
- A monomer to synthesize aromatic polyimides with excellent thermo-mechanical and chemical properties. These polymers find the applications in automotive and electronic industries.
- A capping agent in the development of siloxane-based hybrid materials for potential usage in organic electronics.
- A monomer in the preparation and modification of thin film composite membranes, which are used in water purification, gas separation, and biomedical devices.
- A monomer in the synthesis of pyromellitic diimide-based copolymers as stable cathode active materials for lithium and sodium-ion batteries.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
716.0 °F - closed cup
Flash Point(C)
380 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anastasia Anceschi et al.
Nanomaterials (Basel, Switzerland), 10(3) (2020-03-12)
Cyclodextrin (CD)-based polymers are known to efficiently form molecular inclusion complexes with various organic and inorganic guest compounds. In addition, they also have a great potential as metal complexes because deprotonated hydroxyls can strongly bind metal ions under alkaline conditions.
Shadpour Mallakpour et al.
Amino acids, 43(4), 1605-1613 (2012-02-14)
In the present work, several novel optically active nanostructure poly(amide-imide)s (PAI)s were synthesized via step-growth polymerization reaction of chiral diacids based on pyromellitic dianhydride-derived dicarboxylic acids containing different natural amino acids such as L-alanine, S-valine, L-leucine, L-isoleucine, L-methionine, and L-phenylalanine
High-refractive-index thin films prepared from aminoalkoxysilane-capped pyromellitic dianhydride-titania hybrid materials
Chang C and Chen W
Journal of Polymer Science Part A: Polymer Chemistry, 39(19), 3419-3427 (2001)
Jun-Xia Yu et al.
Environmental science and pollution research international, 20(1), 543-551 (2012-04-25)
The purpose of this research is to use a simple method to prepare magnetic modified biomass with good adsorption performances for cationic ions. The magnetic modified biomass was prepared by two steps: (1) preparation of pyromellitic dianhydride (PMDA) modified biomass
Jun-Xia Yu et al.
Bioresource technology, 110, 160-166 (2012-02-22)
Magnetic pyromellitic dianhydride (PMDA) modified sugarcane bagasse (SCB) was prepared by a situ co-precipitation method. Results showed that the magnetic modified SCB could be recycled easily by an applied magnetic field. Adsorption capacities of the magnetic sorbent for cationic dyes:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic dianhydride 99%](/deepweb/assets/sigmaaldrich/product/structures/418/038/9edd3533-0f32-442c-8a1f-4e154e65c3b5/640/9edd3533-0f32-442c-8a1f-4e154e65c3b5.png)