All Photos(1)
About This Item
Empirical Formula (Hill Notation):
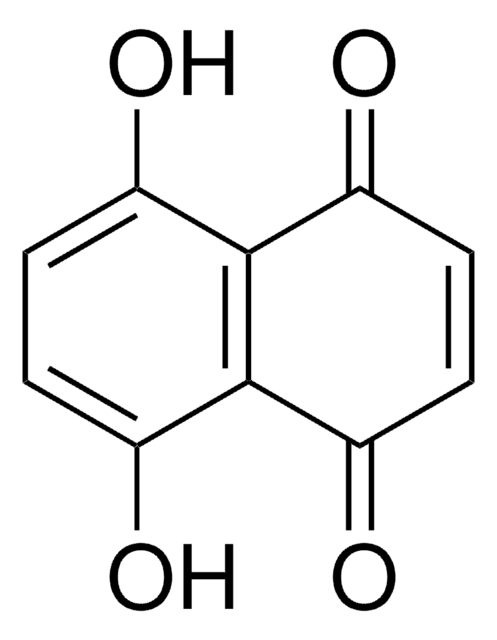
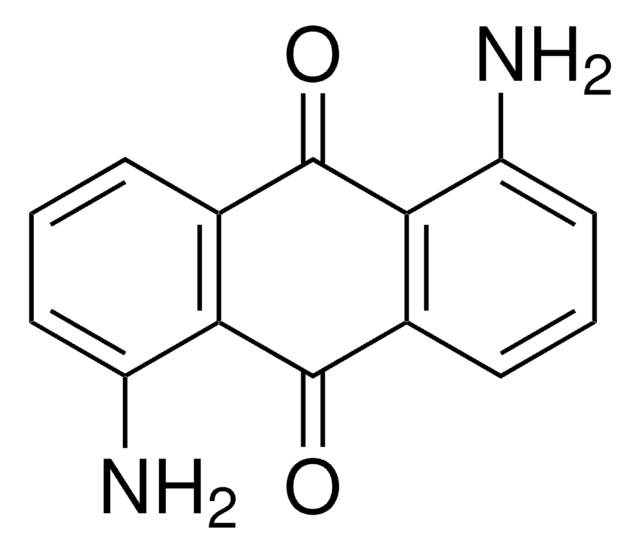
C18H10O4
CAS Number:
Molecular Weight:
290.27
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
mp
350-351 °C (lit.)
SMILES string
Oc1c2C(=O)c3ccccc3C(=O)c2c(O)c4ccccc14
InChI
1S/C18H10O4/c19-15-9-5-1-2-6-10(9)16(20)14-13(15)17(21)11-7-3-4-8-12(11)18(14)22/h1-8,19-20H
InChI key
QECAURYYBPUIFF-UHFFFAOYSA-N
Related Categories
General description
Crystallographic analysis of 6,11-dihydroxy-5,12-naphthacenedione has been reported. Study indicates the presence of (3Z,3′Z)-3,3′-(ethane-1,2-diylidene)bis[isobenzofuran-1(3H)-one] as impurity.
Application
6,11-Dihydroxy-5,12-naphthacenedione was used in the synthesis of a tetracene derivative, 5,6,11,12-tetrachlorotetracene. It was also used for the synthesis of (3Z,3′Z)-3,3′-(ethane-1,2-diylidene)bis[isobenzofuran-1(3H)-one]. It may be used for the following studies:
- Synthesis of self-assembled, chair-shaped dirhenium(I) macrocyclic compounds.
- Synthesis of half-sandwich Ir, Rh-based organometallic molecular boxes.
- As ligand for the synthesis of supramolecular coordination complexes (SCCs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dibyendu Bhattacharya et al.
Inorganic chemistry, 49(22), 10264-10272 (2010-10-16)
Self-assembled, chair-shaped dirhenium(I) macrocyclic compounds featuring the two different bis-chelating quinone dianions (1, L = dhnq(2-); 2, L = dhaq(2-); H(2)dhnq = 6,11-dihydroxy-5,12-naphthacenedione; H(2)dhaq = 1,4-dihydroxy-9,10-anthraquinone) that interface with two fac-Re(CO)(3) cores and a ditopic semirigid N-donor 1,4-bis(5,6-dimethylbenzimidazol-1-ylmethyl)naphthalene (L' =
(3Z, 3'Z)-3, 3'-(Ethane-1, 2-diylidene) bis [isobenzofuran-1 (3H)-one].
Ono K, et al.
Acta Crystallographica Section E, Structure Reports Online, 65(9), 2118-2118 (2009)
5, 6, 11, 12-Tetrachlorotetracene, a tetracene derivative with p-stacking structure: The synthesis, crystal structure and transistor properties.
Chi X, et al.
Organic Electronics, 9(2), 234-240 (2008)
Ying-Feng Han et al.
Dalton transactions (Cambridge, England : 2003), 39(16), 3976-3984 (2010-04-08)
Reactions of [Cp*MCl(mu-Cl)](2) (M = Ir or Rh) with 6,11-dihydroxy-5,12-naphthacenedione (H(2)DHNA) in the presence of base, gave the corresponding binuclear complexes [Cp*(2)M(2)(mu-DHNA)Cl(2)] (M = Ir (1a); M = Rh (1b)), respectively. Treatment of 1a or 1b with bidentate ligands (L)
Synthesis of rhenium-based M 2 LL'-type supramolecular coordination complexes from flexible ligands.
Shankar B, et al.
Journal of Organometallic Chemistry, 743, 109-113 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service