All Photos(1)
About This Item
Empirical Formula (Hill Notation):
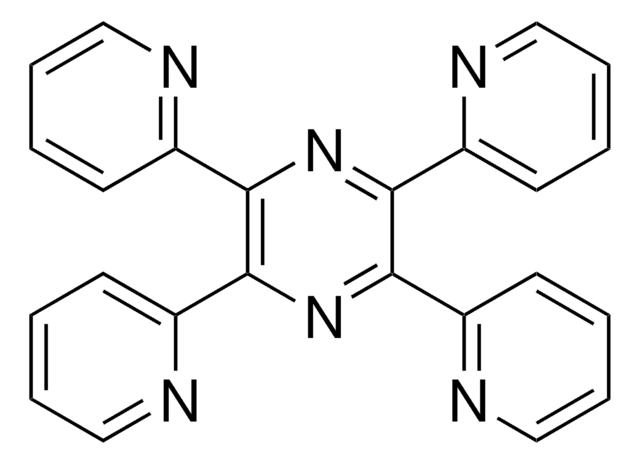
C12H8N6
CAS Number:
Molecular Weight:
236.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
mp
225 °C (dec.) (lit.)
SMILES string
c1ccc(nc1)-c2nnc(nn2)-c3ccccn3
InChI
1S/C12H8N6/c1-3-7-13-9(5-1)11-15-17-12(18-16-11)10-6-2-4-8-14-10/h1-8H
InChI key
JFBIRMIEJBPDTQ-UHFFFAOYSA-N
General description
3,6-Di-2-pyridyl-1,2,4,5-tetrazine (DPTZ) undergoes solvothermal reaction with CuSO4.6H2O and Cu(Ac)2.H2O to afford copper containing coordination polymers. It reacts with enamine derivative of morpholine and 5-,6-,7- and 8-membered cyclic ketones to afford pyridazine derivatives.
3,6-Di-2-pyridyl-1,2,4,5-tetrazine undergoes solid-state reaction with fullerene C60 by high-speed vibration milling technique. It acts as electron-deficient diene in the inverse electron demand Diels Alder reaction.
Application
3,6-Di-2-pyridyl-1,2,4,5-tetrazine may be used in the following studies:
- Preparation of mononuclear, cyclic tetranuclear, and 1-D helical-chain Cu(II) complexes, via metal-assisted hydrolysis.
- Synthesis of novel cyclic tetranuclear ZnII complex.
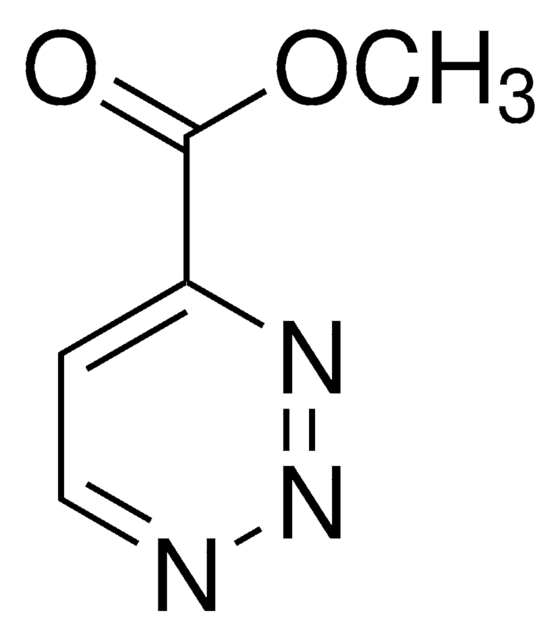
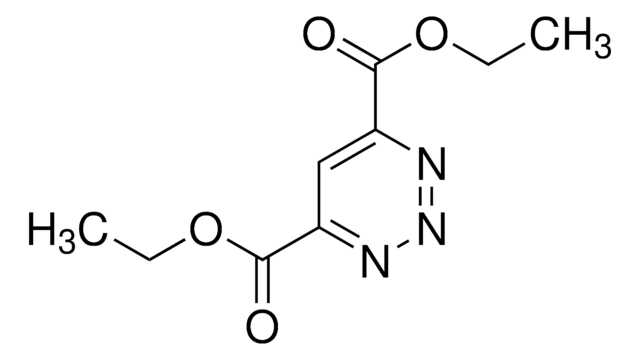
- Synthesis of substituted 3,6-di(2-pyridyl)pyridazines, via inverse electron demand Diels Alder reaction with alkenes or alkynes.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Characterization of Novel Substituted 3, 6-Di (2-pyridyl) pyridazine Metal-Coordinating Ligands.
Hoogenboom R, et al.
European Journal of Organic Chemistry, 2003(24), 4887-4896 (2003)
Synthesis and properties of five unexpected copper complexes with ring-cleavage of 3, 6-di-2-pyridyl-1, 2, 4, 5-tetrazine by one pot in situ hydrothermal reaction.
Cui J, et al.
CrystEngComm, 14(6), 2258-2267 (2012)
Xian-He Bu et al.
Inorganic chemistry, 41(7), 1855-1861 (2002-04-02)
The reactions of 3,6-di-2-pyridyl-1,2,4,5-tetrazine (DPTZ) with different Cu(II) salts generate two new ligands, 2,5-bis(2-pyridyl)-1,3,4-oxodiazole (L(1)) and N,N'-bis(alpha-hydroxyl-2-pyridyl)ketazine (H(2)L(2)), from the metal-assisted hydrolysis of DPTZ, and form three new complexes: a mononuclear complex [Cu(L(1))(2)(H(2)O)(2)] .2ClO(4) (1), a linear coordination polymer [Cu(L(1))(NO(3))(2)](8)
Structure of the Hydration Product of the C60-Di (2-pyridyl)-1, 2, 4, 5-tetrazine Adduct.
Murata Y, et al.
Bulletin of the Chemical Society of Japan, 76(8), 1669-1672 (2003)
Diels-Alder reactions of 3, 6-diphenyl-1, 2, 4, 5-tetrazine and 3, 6-di (2-pyridyl)-1, 2, 4, 5-tetrazine with some 1-morpholinocycloalkenes.
Adnan Atfah M.
Journal of Heterocyclic Chemistry, 26(3), 717-719 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service