391107
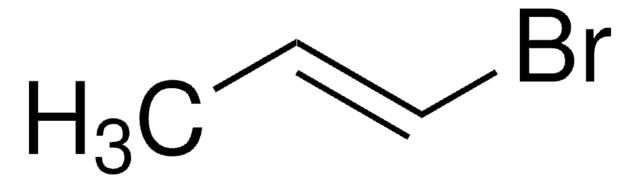
trans-1-Bromo-1-propene
contains copper as stabilizer, 99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH=CHBr
CAS Number:
Molecular Weight:
120.98
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.453 (lit.)
bp
64-65 °C (lit.)
density
1.408 g/mL at 25 °C (lit.)
functional group
alkyl halide
bromo
storage temp.
2-8°C
SMILES string
C\C=C\Br
InChI
1S/C3H5Br/c1-2-3-4/h2-3H,1H3/b3-2+
InChI key
NNQDMQVWOWCVEM-NSCUHMNNSA-N
Related Categories
General description
trans-1-Bromo-1-propene is an alkenyl halide. Product contains copper as stabilizer. Its synthesis from 1,2-dibromopropane has been reported by various researchers. Its IR spectra has been investigated.
Application
trans -1-Bromo-1-propene is suitable for the synthesis of (E)-hex-4-en-2-yn-1-ol. trans -1-Bromo-1-propene ((E)-1-bromo-1-propene) may be used in the synthesis of the following:
- (E)-1,3-dimethyl-3-(prop-1-enyl)indolin-2-one
- 3-alkenyl-Δ3-cephems
- (E)-1-(benzylthio)-1-propene
trans-1-Bromo-1-propene may be used as starting reagent in the synthesis of (+)-trans-isoalliin and (-)-trans-isoalliin.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
5.0 °F - closed cup
Flash Point(C)
-15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alexander M Taylor et al.
Journal of the American Chemical Society, 131(29), 9900-9901 (2009-07-08)
The enantioselective alpha-arylation and alpha-vinylation of oxindoles catalyzed by Pd and a biarylmonophosphine ligand with both axial and phosphorus-based chirogenicity is reported. The resultant quaternary carbon stereocenters are formed in high enantiomeric excess, and the conditions tolerate a range of
cis-trans Isomerization of 1-Bromo-1-propene and Related Compounds.
Harwell, KE and Hatch LF.
Journal of the American Chemical Society, 77(6), 1682-1684 (1955)
The Preparation of Long Chain Alkylamine Hydrochlorides1a.
Murr BL and Lester CT.
Journal of the American Chemical Society, 77(6), 1684-1685 (1955)
H Tanaka et al.
The Journal of organic chemistry, 66(2), 570-577 (2001-06-30)
Synthesis of 3-alkenyl-delta 3-cephems was performed successfully by cross-coupling 3-(trifluoromethylsulfonyloxy or chloro)-delta 3-cephem with alkenyl halides, e.g., vinyl bromide, trans-1-bromo-1-propene, and trans-beta-bromostyrene in an Al/cat.PbBr2/cat.NiBr2(bpy)/NMP (or DMF) system. Reduction of 3-(trifluoromethylsulfonyloxy)-delta 3-cephem into norcephalosporin was also achieved by a similar
Substituted Dienols from Palladium Catalyzed Coupling of Hydroaluminated Enynols with Aryl Iodides.
Crook KE, et al.
Letters in Organic Chemistry, 5(3), 158-164 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service