368954
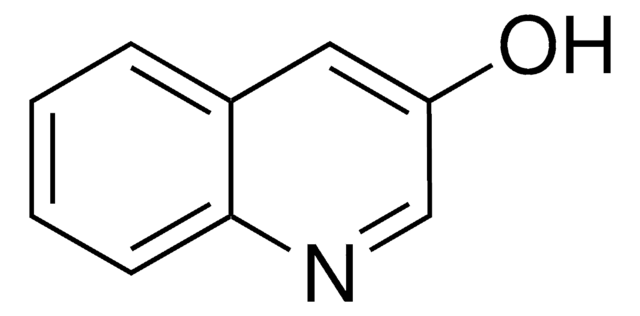
3-Hydroxyisoquinoline
99%
Synonym(s):
3-Isoquinolinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
192-194 °C (lit.)
SMILES string
Oc1cc2ccccc2cn1
InChI
1S/C9H7NO/c11-9-5-7-3-1-2-4-8(7)6-10-9/h1-6H,(H,10,11)
InChI key
GYPOFOQUZZUVQL-UHFFFAOYSA-N
General description
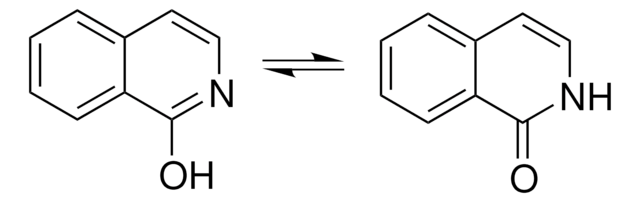
Two excited state proton transfer mechanisms of 3-hydroxyisoquinoline (3HIQ) in cyclohexane and acetic acid has been investigated by time-dependent density functional theory (TDDFT). Spectral and photo physical properties of 3-HIQ in various protic/aprotic solvents were studied. Phototautomerization of 3-HIQ has been reported. Oxo-hydroxy tautomerism and phototautomerism of 3-HIQ has been studied using the matrix-isolation technique.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anna Gerega et al.
The journal of physical chemistry. A, 111(23), 4934-4943 (2007-05-22)
Oxo-hydroxy tautomerism and phototautomerism of 2-quinolinone, 1-isoquinolinone, 3-hydroxyisoquinoline, 2-quinoxalinone, and 4-quinazolinone were studied using the matrix-isolation technique. These compounds contain a benzene ring fused with a heterocyclic ring of 2-pyridinone, 2-pyrazinone, or 4-pyrimidinone. It turned out that direct attachment of
Neeraj Kumar Joshi et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 13(6), 929-938 (2014-04-15)
In the present work we report the spectral and photophysical properties of 3-hydroxyisoquinoline in various protic/aprotic solvents. Our steady state and time resolved fluorescence data indicates that in the monomer form of 3HIQ phototautomerization can take place in the excited
Jiajia Guo et al.
Organic & biomolecular chemistry, 13(4), 1179-1186 (2014-11-28)
An efficient and enantioselective strategy to synthesize benzoindolizidines from α,β-unsaturated amino ketones via domino intramolecular aza-Michael addition/alkylation was developed. These reactions were enabled by cinchona alkaloid-derived quaternary ammonium salts as the phase-transfer catalyst. A variety of benzoindolizidines were prepared in
Jinfeng Zhao et al.
Physical chemistry chemical physics : PCCP, 17(2), 1142-1150 (2014-11-25)
Two excited state proton transfer mechanisms of 3-hydroxyisoquinoline (3HIQ) in cyclohexane and acetic acid (ACID) were investigated based on the time-dependent density functional theory (TDDFT), suggesting a different double-proton transfer mechanism from the one proposed previously (J. Phys. Chem. B
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service