All Photos(1)
About This Item
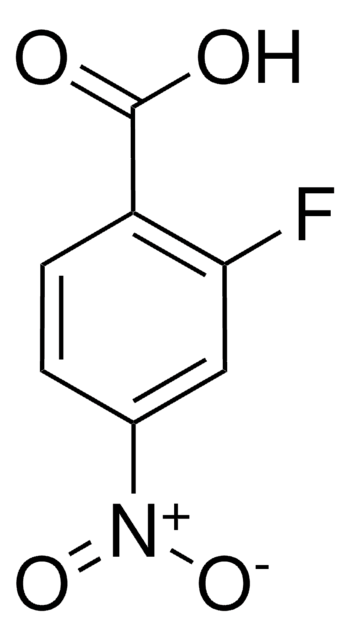
Linear Formula:
FC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
185.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
142-144 °C (lit.)
functional group
carboxylic acid
fluoro
nitro
SMILES string
OC(=O)c1cc(ccc1F)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-6-2-1-4(9(12)13)3-5(6)7(10)11/h1-3H,(H,10,11)
InChI key
ICXSHFWYCHJILC-UHFFFAOYSA-N
General description
2-Fluoro-5-nitrobenzoic acid is reported to react with an aldehyde, isonitrile and a primary amine tethered to a Boc-protected internal amino or hydroxyl nucleophile, to afford the Ugi product.
Application
2-Fluoro-5-nitrobenzoic acid may be used in the synthesis of:
- dibenz[b,f]oxazepin-11(10H)-ones, via the nucleophilic aromatic substitution (SNAr) of fluorine in 2-fluoro-5-nitrobenzoic acid with the OH of various 2-aminophenols on solid support
- synthesis of substituted dibenzazocines on solid support, via SNAr of fluorine in 2-fluoro-5-nitrobenzoic acid with the OH function of the immobilized polysubstituted phenols
- in the synthesis of oxazepines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solid support synthesis of 2-substituted dibenz [b, f] oxazepin-11 (10H)-ones< i> via</i> S< sub> N</sub> Ar methodology on AMEBA resin.
Ouyang X, et al.
Tetrahedron, 55(10), 2827-2834 (1999)
Efficient approach for the diversity-oriented synthesis of macro-heterocycles on solid-support.
Giulianotti M and Nefzi A.
Tetrahedron Letters, 44(28), 5307-5309 (2003)
MCC/SN Ar methodology. Part 2: Novel three-step solution phase access to libraries of benzodiazepines.
Tempest P, et al.
Tetrahedron Letters, 44(9), 1947-1950 (2003)
Wim Meutermans et al.
ChemMedChem, 1(11), 1164-1194 (2006-09-20)
Drug discovery has long suffered from the difficulty of having to place pharmacophoric groups in just the right spatial arrangement to elicit the desired biological response. Although some molecule classes have been discovered that seem to be privileged structures for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service