361046
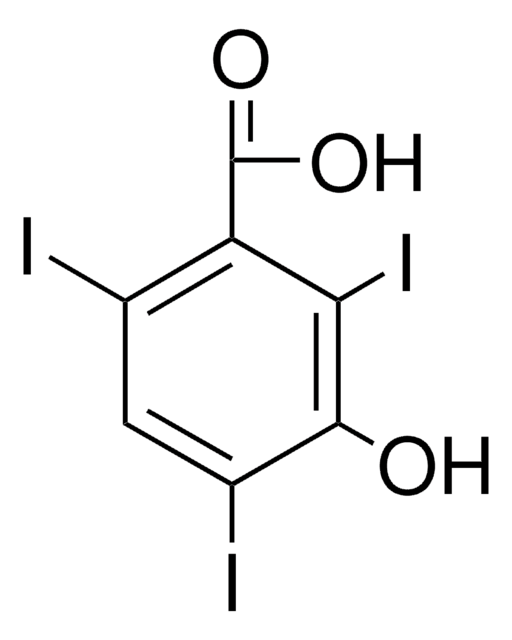
α-Ethyl-3-hydroxy-2,4,6-triiodohydrocinnamic acid
97%
Synonym(s):
Iophenoxic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
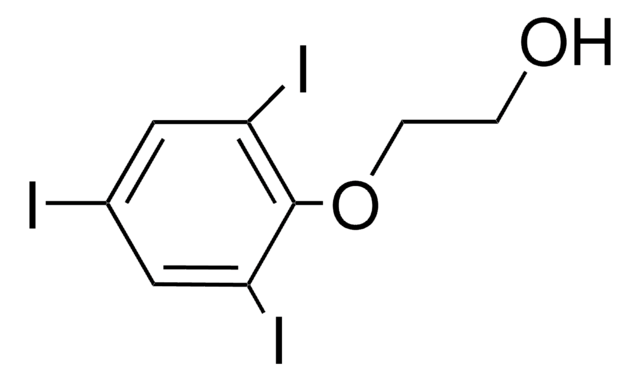
HOC6H(I)3CH2CH(C2H5)CO2H
CAS Number:
Molecular Weight:
571.92
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
146-148 °C (lit.)
SMILES string
CCC(Cc1c(I)cc(I)c(O)c1I)C(O)=O
InChI
1S/C11H11I3O3/c1-2-5(11(16)17)3-6-7(12)4-8(13)10(15)9(6)14/h4-5,15H,2-3H2,1H3,(H,16,17)
InChI key
GOIQOQCNFWYSTQ-UHFFFAOYSA-N
General description
α-Ethyl-3-hydroxy-2,4,6-triiodohydrocinnamic acid (Iophenoxic acid ) is an organic, iodine-containing compound. Iophenoxic acid is an iodinated radiocontrast agent, its clinical use has been withdrawn due to its exceptionally long half-life in the body, since it has high-affinity binding to human serum albumin (HSA). Structural basis of its interaction with HSA has been evaluated.
Application
α-Ethyl-3-hydroxy-2,4,6-triiodohydrocinnamic acid (iophenoxic acid ) was used as marker in “bait acceptance” studies conducted on various animal species. It was used in the direct quantitation of iophenoxic acid in porcine serum samples by a liquid chromatographic-electrospray ionization mass spectrometric technique.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Carlo Bertucci et al.
Farmaco (Societa chimica italiana : 1989), 58(9), 901-908 (2003-09-19)
The binding of two cholecystographic agents to human serum albumin (HSA) was evaluated by means of two different complementary methodologies. In particular, the inhibition of drug HSA binding caused by iopanoic- and iophenoxic-acid was investigated by circular dichroism (CD) and
J Hadidian et al.
Journal of wildlife diseases, 25(1), 1-9 (1989-01-01)
In summer 1986, a study was conducted to evaluate raccoon (Procyon lotor) acceptance of oral baits that could be used for rabies vaccination. One thousand wax-coated sponge bait cubes were filled with 5 mg of a seromarker (iophenoxic acid), placed
U Kragh-Hansen
Molecular pharmacology, 34(2), 160-171 (1988-08-01)
The relations between the single high affinity binding sites for azapropazone, phenylbutazone, chlorpropamide, sulfathiazole, and iophenoxate and the binding regions of human serum albumin represented by the marker ligands diazepam, phenol red, salicylate, and warfarin were examined by a series
C T Eason et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(2), 185-189 (1992-02-01)
1. The comparative plasma pharmacokinetics of two organic iodine-containing compounds were evaluated in the goat for their suitability as markers in wildlife studies. 2. After oral administration of a single dose, the plasma elimination half-life for iopanoic acid was considerably
A Jones
Journal of chromatography. B, Biomedical applications, 654(2), 293-296 (1994-04-01)
Iophenoxic acid (IPA), a marker used to investigate the feeding behaviour of bait-consuming animals has previously been indirectly determined by measuring protein-bound iodine levels in serum or plasma. For the first time a method is reported for the direct determination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service