330884
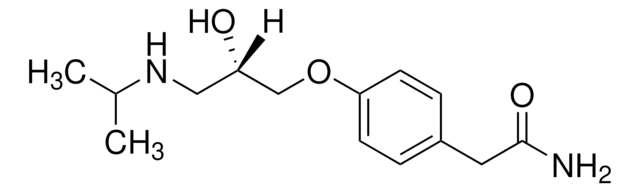
(R)-(+)-Atenolol
99%
Synonym(s):
(+)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHNHCH2CH(OH)CH2OC6H4CH2CONH2
CAS Number:
Molecular Weight:
266.34
MDL number:
UNSPSC Code:
12352112
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D +16°, c = 1 in 1 M HCl
mp
148-152 °C (lit.)
SMILES string
CC(C)NC[C@@H](O)COc1ccc(CC(N)=O)cc1
InChI
1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)/t12-/m1/s1
InChI key
METKIMKYRPQLGS-GFCCVEGCSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Joni Agustian et al.
Chirality, 29(7), 376-385 (2017-04-26)
As the (R)-enantiomer of racemic atenolol has no β-blocking activity and no lack of side effects, switching from the racemate to the (S)-atenolol is more favorable. Transesterification of racemic atenolol using free enzymes investigated as a resource to resolve the
Joni Agustian et al.
Chirality, 29(12), 847-853 (2017-10-01)
Kinetic resolution of (R,S)-atenolol is a faster strategy to produce (S)-atenolol. Since this racemate is a less soluble compound, resolution of its ester offers high concentrations in the process. A good analytical method is required to observe the enantiomer concentrations.
Kevin F Morris et al.
Chemical physics, 457, 133-146 (2015-08-11)
Molecular dynamics simulations and NMR spectroscopy were used to compare the binding of two β-blocker drugs to the chiral molecular micelle poly-(sodium undecyl-(L)-leucine-valine). The molecular micelle is used as a chiral selector in capillary electrophoresis. This study is part of
Ulisse Garbin et al.
Mediators of inflammation, 2008, 367590-367590 (2008-04-26)
The endothelium plays a key role in the development of atherogenesis and its inflammatory and proliferative status influences the progression of atherosclerosis. The aim of this study is to compare the effects of two beta blockers such as nebivolol and
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service