31460
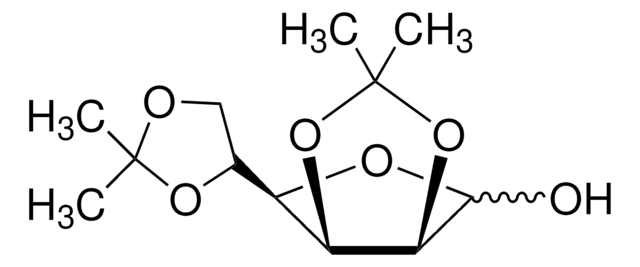
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose
purum, ≥98.0% (TLC)
Synonym(s):
D-Glucose diacetonide, Diacetone-D-glucose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H20O6
CAS Number:
Molecular Weight:
260.28
Beilstein:
84386
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (TLC)
optical activity
[α]20/D −11.5±1°, c = 5% in ethanol
mp
110-111 °C (lit.)
SMILES string
CC1(C)OC[C@@H](O1)[C@H]2O[C@@H]3OC(C)(C)O[C@@H]3[C@H]2O
InChI
1S/C12H20O6/c1-11(2)14-5-6(16-11)8-7(13)9-10(15-8)18-12(3,4)17-9/h6-10,13H,5H2,1-4H3/t6-,7+,8-,9-,10-/m1/s1
InChI key
KEJGAYKWRDILTF-JDDHQFAOSA-N
Looking for similar products? Visit Product Comparison Guide
Other Notes
Important protected derivative of glucose; the 3-OH group can be directly manipulated, the 5,6-O-isopropylidene protection is selectively cleavable; oxidation and reduction of the 3-OH leads to an allofuranose derivative; review
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S C Hung et al.
Carbohydrate research, 331(4), 369-374 (2001-06-12)
A practical route toward the synthesis of 6-deoxy-L-idose and L-acovenose from 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose is described. Key steps include the stereoselective hydrogenation of 6-deoxy-1,2:3,5-di-O-isopropylidene-alpha-D-xylo-hex-5-enofuranose, regioselective protection of 6-deoxy-1,2-O-isopropylidene-beta-L-idofuranose at 0-5, and epimerisation of 6-deoxy-5-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-beta-L-idofuranose at C-3.
Guylaine M Defossemont et al.
Carbohydrate research, 338(6), 563-565 (2003-04-02)
The synthesis and characterisation of a novel chiral bicyclic oxacaprolactone is reported. The choice of diisopropylidene-D-glucose as a starting material allowed selective introduction of the synthetic equivalent necessary for the formation of the seven-membered ring of the lactone, i.e., one
Leonardo Guimarães de Oliveira et al.
Meat science, 155, 50-60 (2019-05-11)
The aim of this study was to determine the extent to which calpastatin (CASN) variants (based on two chromatographic peaks; CASN-P1 and CASN-P2) explain variation in μ-calpain autolysis, protein degradation, and changes in the sarcoplasmic proteome observed during postmortem aging
Yoon-Suk Kang et al.
Infection and immunity, 87(8) (2019-06-05)
Brucella is an intracellular bacterial pathogen that causes chronic systemic infection in domesticated livestock and poses a zoonotic infectious risk to humans. The virulence of Brucella is critically dependent on its ability to replicate and survive within host macrophages. Brucella
S. Iacono et al.
Organic Syntheses, 64, 57-57 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(3AR,5S,6S,6AR)-5-((R)-2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)-2,2-DIMETHYLTETRAHYDROFURO[3,2-D][1,3]DIOXOL-6-OL AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/241/825/1c695a85-5c36-42d3-806a-30876a4dabac/640/1c695a85-5c36-42d3-806a-30876a4dabac.png)