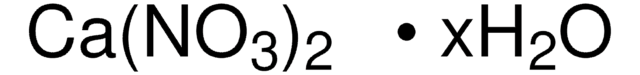288640
Manganese(II) nitrate hydrate
98%
Synonym(s):
Manganese(2+) dinitrate hydrate, Manganous dinitrate hydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
Mn(NO3)2·xH2O
CAS Number:
Molecular Weight:
178.95 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
composition
Degree of hydration, 4-6
storage temp.
2-8°C
SMILES string
O.[Mn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mn.2NO3.H2O/c;2*2-1(3)4;/h;;;1H2/q+2;2*-1;
InChI key
HBTFASPVVFSRRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Manganese(II) nitrate hydrate is an inorganic salt highly soluble in water. It can be used as a source of manganese ions in the synthesis of various manganese-containing materials for batteries and supercapacitor applications. Additionally, it also serves as a safe, cost-effective, and efficient electrolyte for rapid energy storage applications.
Application
Manganese(II) nitrate hydrate can be used as:
- A precursor for the synthesis of manganese dioxide (MnO2), which is a common cathode material in primary alkaline batteries.
- A precursor in the synthesis of manganese dioxide (MnO2) for use in flexible micro supercapacitors. MnO2 exhibits high specific capacitance and excellent cycling stability, making it suitable for rapid energy storage applications.
- A manganese source in the colloidal solution combustion synthesis process to produce 3D δ-MnO2 nanostructures with ultra-large mesopores used in the fabrication of high-performance lithium-ion battery anodes.
- A precursor in the microwave-aided fabrication of calcium-substituted dysprosium perovskite manganite oxide nanocomposites (DyMnO3) for supercapacitor applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Separation of rare earths from transition metals by liquid-liquid extraction from a molten salt hydrate to an ionic liquid phase
Rout A and Binnemans K
Dalton Transactions, 43(8), 3186-3195 (2014)
Jaechan Ryu et al.
Nature communications, 9(1), 3715-3715 (2018-09-15)
Aluminum-air batteries are promising candidates for next-generation high-energy-density storage, but the inherent limitations hinder their practical use. Here, we show that silver nanoparticle-mediated silver manganate nanoplates are a highly active and chemically stable catalyst for oxygen reduction in alkaline media.
Thermal characteristics of manganese (II) nitrate hexahydrate as a phase change material for cooling systems
Nagano K, et al.
Applied Thermal Engineering, 23(2), 229-241 (2003)
Reactive ceramics of CeO2-MOx (M= Mn, Fe, Ni, Cu) for H2 generation by two-step water splitting using concentrated solar thermal energy
Kaneko H, et al.
Energy, 32(5), 656-663 (2007)
Breno R Barrioni et al.
Journal of colloid and interface science, 547, 382-392 (2019-04-12)
Bioactive glass nanoparticles (BGNPs) are of great interest in tissue engineering as they possess high dissolution rate and capability of being internalized by cells, releasing their dissolution products with therapeutic benefits intracellularly. A modified Stöber process can be applied to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service