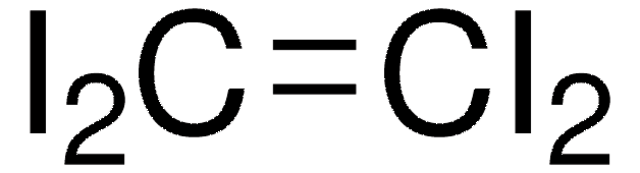282286
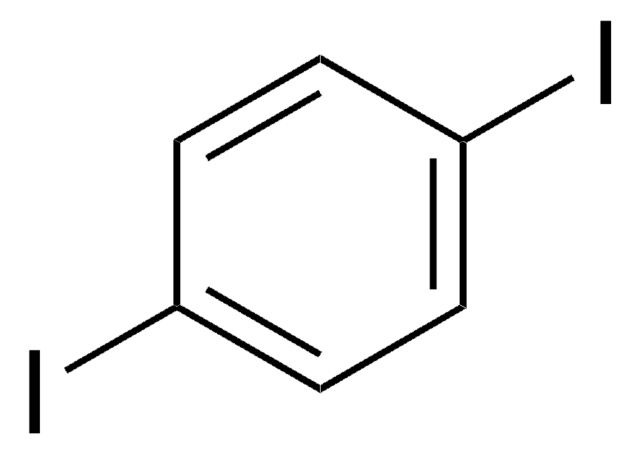
1,4-Diiodotetrafluorobenzene
98%
Synonym(s):
1,2,4,5-Tetrafluoro-3,6-diiodobenzene, 1,4-Diiodo-2,3,5,6-tetrafluorobenzene, 3,6-Diiodo-1,2,4,5-tetrafluorobenzene, Tetrafluoro-1,4-diiodobenzene, p-Diiodoperfluorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6F4I2
CAS Number:
Molecular Weight:
401.87
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
108-110 °C (lit.)
functional group
fluoro
SMILES string
Fc1c(F)c(I)c(F)c(F)c1I
InChI
1S/C6F4I2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI key
VIXRAZODEODOJF-UHFFFAOYSA-N
General description
1,4-Diiodotetrafluorobenzene forms trimeric complexes as new, halogen-bonded mesogens with two molecules of alkoxystilbazole. The electron density study of the halogen-bonded complex of 4,4′-dipyridyl-N,N′-dioxide with 1,4-diiodotetrafluorobenzene at 90K has been reported.
1,4-Diiodotetrafluorobenzene is a ditopic perfluorinated iodobenzene used for cocrystallization of halopyridinium salts.
1,4-Diiodotetrafluorobenzene is a ditopic perfluorinated iodobenzene used for cocrystallization of halopyridinium salts.
Application
1,4-Diiodotetrafluorobenzene has been used as the halogen bonding donor during the preparation of phosphorescent cocrystal with polycyclic aromatic hydrocarbons.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Halogen and hydrogen bond motifs in ionic cocrystals derived from 3-halopyridinium halogenides and perfluorinated iodobenzenes
Lidija P et al.
Crystal Growth & Design, 21, 6044-6050 (2021)
Jay Quentin et al.
Molecules (Basel, Switzerland), 25(4) (2020-02-23)
The halogen-bond (X-bond) donors 1,3- and 1,4-diiodotetrafluorobenzene (1,3-di-I-tFb and 1,4-di-I-tFb, respectively) form cocrystals with trans-1,2-bis(2-pyridyl)ethylene (2,2'-bpe) assembled by N···I X-bonds. In each cocrystal, 2(1,3-di-I-tFb)·2(2,2'-bpe) and (1,4-di-I-tFb)·(2,2'-bpe), the donor molecules support the C=C bonds of 2,2'-bpe to undergo an intermolecular [2+2]
Mesogenic, trimeric, halogen-bonded complexes from alkoxystilbazoles and 1, 4-diiodotetrafluorobenzene.
<BIG>Bruce DW, et al. </BIG>
New. J. Chem., 32(3), 477-482 (2008)
Phosphorescent co-crystal assembled by 1, 4-diiodotetrafluorobenzene with carbazole based on C-Ip halogen bonding.
<BIG>Gao HY, et al. </BIG>
Journal of Materials Chemistry, 22(12), 5336-5343 (2012)
Jiehao Fu et al.
iScience, 23(3), 100965-100965 (2020-03-22)
Here we introduce a σ-hole-containing volatile solid additive, 1, 4-diiodotetrafluorobenzene (A3), in PM6:Y6-based OSCs. Aside from the appropriate volatility of A3 additive, the synergetic halogen interactions between A3 and photoactive matrix contribute to more condensed and ordered molecular arrangement in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service