282103
Triton™ X-100 reduced
Synonym(s):
Polyoxyethylene (10) isooctylcyclohexyl ether
About This Item
Recommended Products
Quality Level
refractive index
n20/D 1.473 (lit.)
density
1.029 g/mL at 25 °C (lit.)
SMILES string
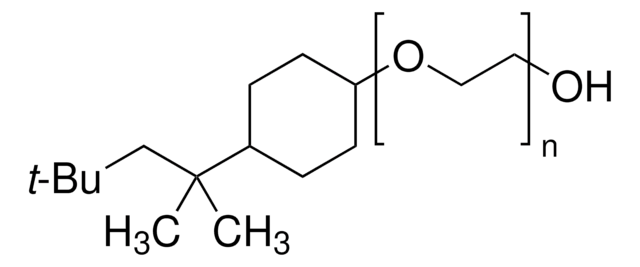
CC(C)(C)CC(C)(C)C1CCC(CC1)OCCOCCOCCOCCOCCOCCOCCO
InChI
1S/C28H56O8/c1-27(2,3)24-28(4,5)25-6-8-26(9-7-25)36-23-22-35-21-20-34-19-18-33-17-16-32-15-14-31-13-12-30-11-10-29/h25-26,29H,6-24H2,1-5H3
InChI key
QQJNBKDKLMCALZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
In biochemical and cell biology research, Triton™ X-100 is instrumental in solubilizing membrane-bound proteins and isolating lipid rafts. Its unique properties allow for the preservation of the native conformation of proteins obtained from cellular membranes in solution. Triton™ X-100 reduced is derived from the full hydrogenation of the benzene moiety of TX-100 to a cyclohexane derivative. This modified version, RTX-100, has demonstrated potential in enhancing enzyme digestion and influencing the photoisomerization of bacteriorhodopsin, showcasing its versatility and utility in advanced research applications.
Application
- as a component of LB-TT for the extraction of total protein from rat brains
- in ADP-Glo assay and Cytophos adenosine triphosphatase (ATPase) assay
- in phosphate-buffered saline (PBS) solution for the permeabilization of fibroblasts in 5′ ethynyl uridine staining, immunofluorescence, and immunolabeling
Features and Benefits
- Non-ionic surfactant
- Reduced polyoxyethylene content (~10)
- Improves solubility and dispersibility of substances
- Excellent wetting properties
- Enhances emulsification
- High purity product for research applications
Other Notes
Legal Information
comparable product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service