270822
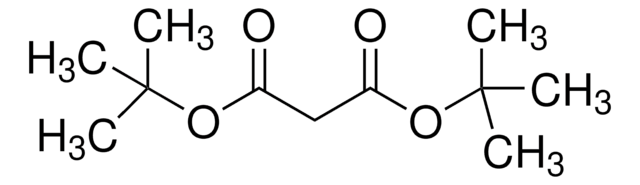
Di-tert-butyl acetylenedicarboxylate
98%
Synonym(s):
2-Butynedioic acid di-tert-butyl ester, Di-tert-butyl 2-butynedioate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3COCOC≡CCOOC(CH3)3
CAS Number:
Molecular Weight:
226.27
Beilstein:
1957547
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
80-82 °C/0.05 mmHg (lit.)
mp
33-37 °C (lit.)
functional group
ester
SMILES string
CC(C)(C)OC(=O)C#CC(=O)OC(C)(C)C
InChI
1S/C12H18O4/c1-11(2,3)15-9(13)7-8-10(14)16-12(4,5)6/h1-6H3
InChI key
FBCRUXRGQFLOMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The cross-cyclotrimerization of di-tert-butyl acetylenedicarboxylate, silylacetylenes and acrylamides was studied with cationic rhodium(I)/(R)-tol-binap complex as catalyst. Glycosyl azides were subjected to 1,3-dipolar cycloaddition with di-tert-butyl acetylenedicarboxylate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W Bröder et al.
Carbohydrate research, 249(1), 221-241 (1993-10-18)
Glycosyl azides provide reliable anomeric protection stable to conditions for hydrolytic removal of ester groups, for reductive opening or release of acetalic diol protection, for the introduction of ether-type protection, and for glycosylation processes. The utility of this anomeric protection
Jun Hara et al.
Angewandte Chemie (International ed. in English), 53(11), 2956-2959 (2014-02-08)
It has been established that a cationic rhodium(I)/(R)-tol-binap complex catalyzes the cross-cyclotrimerization of silylacetylenes, di-tert-butyl acetylenedicarboxylates, and acrylamides with excellent chemo-, regio-, and enantioselectivities. Unsymmetrical alkynoates can also be employed in place of di-tert-butyl acetylenedicarboxylate for this process, but with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service