258598
4-Ethoxyphenol
99%
Synonym(s):
Hydroquinone monoethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OC6H4OH
CAS Number:
Molecular Weight:
138.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
121 °C/9 mmHg (lit.)
mp
64-67 °C (lit.)
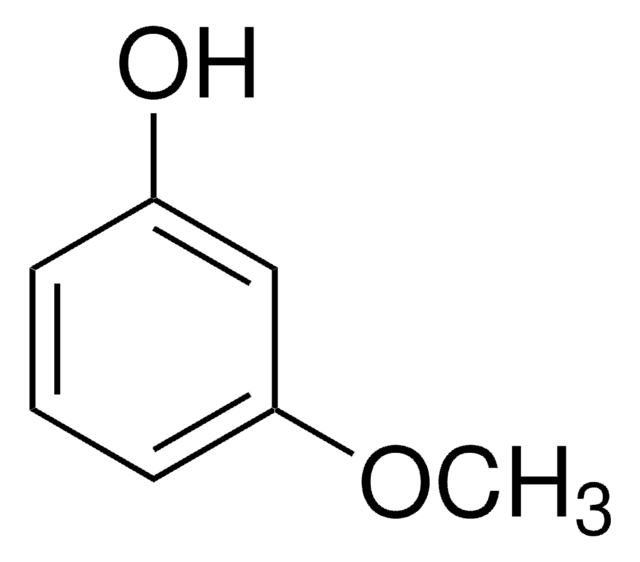
SMILES string
CCOc1ccc(O)cc1
InChI
1S/C8H10O2/c1-2-10-8-5-3-7(9)4-6-8/h3-6,9H,2H2,1H3
InChI key
LKVFCSWBKOVHAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Ethoxyphenol is the major dehalogenated product formed during H2O2-driven microperoxidase-8-catalyzed dehalogenation of 4-fluorophenol.
Application
4-Ethoxyphenol has been used as substrate to evaluate kinetic constant for the monophenolase activity of mushroom tyrosinase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A M Osman et al.
Proceedings of the National Academy of Sciences of the United States of America, 94(9), 4295-4299 (1997-04-29)
The results of this study report the H2O2-driven microperoxidase-8 (MP8)-catalyzed dehalogenation of halophenols such as 4-fluorophenol, 4-chlorophenol, 4-bromophenol, and 2-fluorophenol in alcoholic solvents. In methanol, the conversion of the para-halophenols and 2-fluorophenol to, respectively, 4-methoxyphenol and 2-methoxyphenol, as the major
J C Espín et al.
European journal of biochemistry, 267(5), 1270-1279 (2000-02-26)
This paper reports a quantitative study of the effect of ring substituents in the 1-position of the aromatic ring on the rate of monophenol hydroxylation and o-diphenol oxidation catalyzed by tyrosinase. A possible correlation between the electron density of the
Cynthia D Selassie et al.
Journal of medicinal chemistry, 48(23), 7234-7242 (2005-11-11)
In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop the following
Alfonso Pérez-Garrido et al.
Bioorganic & medicinal chemistry, 17(2), 896-904 (2008-12-06)
This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the molecular structure of the compounds under investigation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service