258083
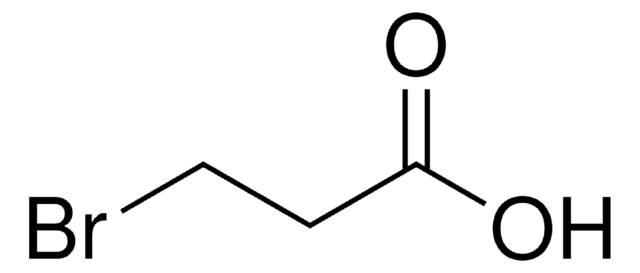
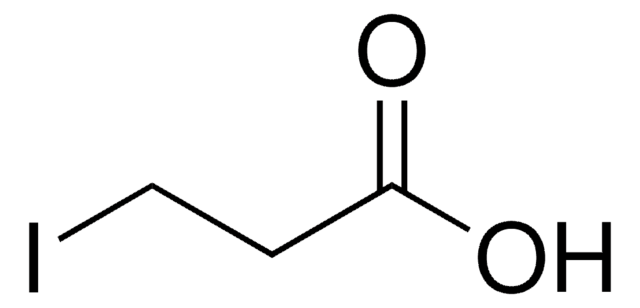
4-Bromobutyric acid
98%
Synonym(s):
4-Bromobutanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)3COOH
CAS Number:
Molecular Weight:
167.00
Beilstein:
1743056
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
reaction suitability
reagent type: cross-linking reagent
bp
128-131 °C/11 mmHg (lit.)
mp
30-31 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
BrCCCC(O)=O
InChI
1S/C4H7BrO2/c5-3-1-2-4(6)7/h1-3H2,(H,6,7)
InChI key
GRHQDJDRGZFIPO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Montanari et al.
Carbohydrate polymers, 221, 209-220 (2019-06-23)
Hyaluronan (HA) is among the most used biopolymers for viscosupplementation and dermocosmetics. However, the current injectable HA-based formulations present relevant limitations: I) unmodified HA is quickly degraded by endogenous hyaluronidases (HAase), resulting in short lasting properties; II) cross-linked HA, although
E Montanari et al.
Journal of controlled release : official journal of the Controlled Release Society, 326, 1-12 (2020-06-20)
Intracellular pathogens are a critical challenge for antimicrobial therapies. Staphylococcus aureus (S. aureus) causes approximately 85% of all skin and soft tissue infections in humans worldwide and more than 30% of patients develop chronic or recurrent infections within three months
Elita Montanari et al.
Advanced healthcare materials, 7(12), e1701483-e1701483 (2018-04-27)
Staphylococcus aureus is one of the most significant human pathogens that is frequently isolated in a wide range of superficial and systemic infections. The ability of S. aureus to invade and survive within host cells such as keratinocytes and host
Amelia C McCue et al.
Photochemistry and photobiology, 94(3), 545-551 (2018-01-30)
Light-responsive compounds have been used to manipulate biological systems with spatial and temporal control of the event of interest. Illumination of alkylcobalamins with green light (>500 nm) produces carbon-centered radicals, which have been demonstrated to effectively cause DNA damage. Molecules that
Renwu Li et al.
Bioorganic & medicinal chemistry, 28(7), 115356-115356 (2020-02-19)
Past few years have seen an active pursuit of the inhibitors for the deacylation catalyzed by the seven human sirtuins (i.e. SIRT1-7) as valuable chemical biological/pharmacological probes of this enzymatic deacylation and lead compounds for developing novel therapeutics for human
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service