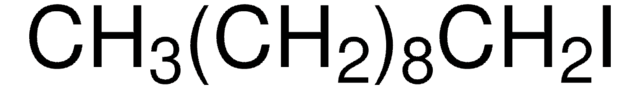All Photos(1)
About This Item
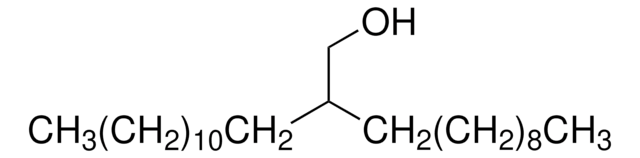
Linear Formula:
CH3(CH2)11I
CAS Number:
Molecular Weight:
296.23
Beilstein:
1738943
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.484 (lit.)
bp
159-160 °C/15 mmHg (lit.)
mp
−3 °C (lit.)
density
1.201 g/mL at 25 °C (lit.)
functional group
alkyl halide
iodo
SMILES string
CCCCCCCCCCCCI
InChI
1S/C12H25I/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-12H2,1H3
InChI key
GCDPERPXPREHJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
SmI2-mediated coupling of 1-iodododecane and 2-octanone in the presence of LiBr, LiCl and hexamethylphosphoramide (HMPA) has been studied.
Application
1-Iodododecane has been used in the preparation of:
- dodecylated graphite
- alkyl functionalized single walled carbon nanotubes (SWNTs)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of SmI2 with alkyl halides and ketones: Inner-sphere vs outer-sphere electron transfer in reactions of Sm (II) reductants.
Miller RS, et al.
Journal of the American Chemical Society, 122(32), 7718-7722 (2000)
Robin E Anderson et al.
Journal of nanoscience and nanotechnology, 7(10), 3436-3440 (2008-03-12)
Single walled carbon nanotubes (SWNTs) may be made soluble in a range of organic solvents without sidewall functionalization via their reduction by Na/Hg amalgam in the presence of dibenzo-18-crown-6. The [Na(dibenzo-18-crown-6)]n[SWNT] complex has been characterized by UV-Visible, Raman, and AFM
Minhuan Li et al.
Science advances, 6(27), eaaw8938-eaaw8938 (2020-07-14)
Most systems have more than two stable crystalline states in the phase diagram, which is known as polymorphism. Crystallization in such a system is often under strong influence of competing orderings linked to those crystals. However, how such competition affects
Functionalization of potassium graphite.
Soma Chakraborty et al.
Angewandte Chemie (International ed. in English), 46(24), 4486-4488 (2007-05-05)
Martha Kafetzi et al.
Polymers, 13(3) (2021-01-27)
In this work, the synthesis and the aqueous solution self-assembly behavior of novel partially hydrophobically modified poly(2-(dimethylamino) ethyl methacrylate)-b-poly(oligo(ethylelene glycol) methyl ether methacrylatetabel) pH and temperature responsive random diblock copolymers (P(DMAEMA-co-Q6/12DMAEMA)-b-POEGMA), are reported. The chemical modifications were accomplished via quaternization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service