23261
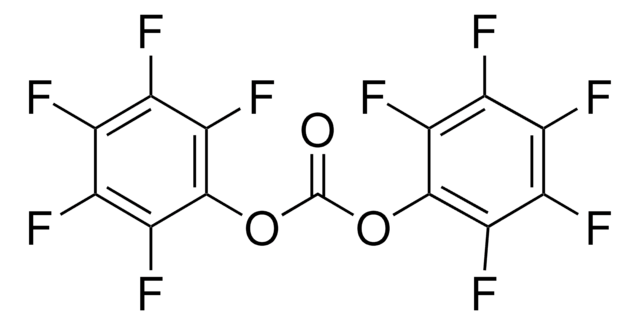
Trichloromethyl chloroformate
≥97.0% (GC)
Synonym(s):
TCF, Diphosgene, di-Phosgene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCOOCCl3
CAS Number:
Molecular Weight:
197.83
Beilstein:
970225
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
refractive index
n20/D 1.458
bp
20 °C/10 mmHg (lit.)
density
1.639 g/mL at 20 °C
functional group
chloro
storage temp.
2-8°C
SMILES string
ClC(=O)OC(Cl)(Cl)Cl
InChI
1S/C2Cl4O2/c3-1(7)8-2(4,5)6
InChI key
HCUYBXPSSCRKRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Cyclic carbamimidates using a monophosphine gold(i) catalyst
- N-Alkenyl and cycloalkyl carbamates as dual acting histamine H3 and H4 receptor ligands
- Prostate-specific membrane antigen-targeted anticancer prodrugs
- Potential west nile virus protease inhibitors
- Antibody-drug conjugates (ADCs)
- Erythromycin A derivatives
Trichloromethyl chloroformate (TCF) is an effective alternative to phosgene. It can react with amines, amino acids and amino alcohols to give the corresponding isocyanates, isocyanato acid chlorides and isocyanato chloroformates.
TCF can also be used:
TCF can also be used:
- To synthesize N-carboxy α-amino acid anhydrides.
- As an acylating agent to synthesize oxazolidinones from α-amino alcohols.
- As a dehydrating agent to synthesize aromatic diisocyanides in the presence of triethylamine.
Other Notes
Easy to handle substitute for phosgene; In-situ charcoal-catalyzed decomposition to phosgene and reaction with amino acids to N-carboxy anhydrides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K. Kunita et al.
Organic Syntheses, 59, 195-195 (1980)
R. Katakai et al.
The Journal of Organic Chemistry, 50, 715-715 (1985)
Synthesis of some aromatic diisocyanides with trichloromethyl chloroformate.
Efraty A, et al.
The Journal of Organic Chemistry, 45(20), 4059-4061 (1980)
An improved rapid method for the synthesis of N-carboxy. alpha.-amino acid anhydrides using trichloromethyl chloroformate.
Katakai R and Iizuka Y
The Journal of Organic Chemistry, 50(5), 715-716 (1985)
Trichloromethyl chloroformate. Reaction with amines, amino acids, and amino alcohols.
Kurita K, et al.
The Journal of Organic Chemistry, 41(11), 2070-2071 (1976)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service